Distribution, ecological niche modelling and conservation assessment of the Peruvian Night Monkey (Mammalia: Primates: Aotidae: Aotus miconax Thomas, 1927) in northeastern Peru, with notes on the distributions of Aotus spp.
Sam Shanee 1, Nestor Allgas 2, Noga Shanee 3 & Nicola Campbell 4
1,3,4 Neotropical Primate Conservation, 23 Portland Road, Manchester M32 0PH, United Kingdom
1,2,3 Asociación Neotropical Primate Conservation Perú, 1187 Carretera Fernando Belaunde Terry, La Esperanza, Yambrasbamba, Perú
2,3 Universidad Nacional Mayor de San Marcos, Facultad de Ciencias Biológicas, Av. Universitaria/Av. Germán Amézaga s/n, Edificio Jorge Basadre, Ciudad Universitaria, Lima, Perú
1 sam@neoprimate.org (corresponding author), 2 nestor.allgas@gmail.com, 3 nogashanee@neoprimate.org,
4 nicolacampbell222@gmail.com
doi: http://dx.doi.org/10.11609/JoTT.o4184.6947-64 | ZooBank: urn:lsid:zoobank.org:pub:FE8DF7B0-FF68-47BC-BF6B-7144F24701DE
Editor: Mewa Singh, University of Mysore, Mysuru, India. Date of publication: 26 March 2015 (online & print)
Manuscript details: Ms # o4184 | Received 12 November 2014 | Final received 09 March 2015 | Finally accepted 10 March 2015
Citation: Shanee, S., N. Allgas, N. Shanee & N. Campbell (2015). Distribution survey, ecological niche modelling and conservation assessment of the Peruvian Night Monkey: Aotus Miconax Thomas, 1927 (Mammalia: Primates: Aotidae) in north-eastern Peru, with notes on the distributions of Aotus spp. Gray, 1870. Journal of Threatened Taxa 7(3): 6947–6964; http://dx.doi.org/10.11609/JoTT.o4184.6947-64
Copyright: © Shanee et al. 2015. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
Funding: This work was funded by Neotropical Primate Conservation and Community Conservation thanks to grants from: American Society of Primatologists, Primate Society of Great Britain, Primate Conservation Inc., The National Geographic Society, International Primate Protection League, Wild Futures, Apenhuel Primate Conservation Trust, Le Conservatoire pour la Protection des Primates, Margot Marsh Biodiversity Foundation.
Competing Interest: The authors declare no competing interests.
Acknowledgements: We wish to thank A. Peralta, F. Guerra-Vasquez, A. Alarcon-Pardo, F. Briceno, J. Sanchez, K. Ramirez and N. Rojas-Pilco for their help in the field. Also M. Leo-Luna, J. Tello, W. Palomino, W. Guzman, L. Rimarachin, J. Vermeer, C. Flores, M. Epiquien, L. Durand, A. Zamora, Y. Julca, M. Diaz and IKAMA Peru. This work was funded by Neotropical Primate Conservation thanks to grants from American Society of Primatologists, Primate Society of Great Britain, Primate Conservation Inc, The National Geographic Society, International Primate Protection League - UK, The Monkey Sanctuary Trust/Wild Futures, Apenhuel Primate Conservation Trust, Le Conservatoire pour la Protection des Primates, International Primate Protection League - US, Community Conservation, and the Margot Marsh Biodiversity Foundation. We would like to thank the InstitutoNacional de RecursosNaturales/Ministerio de Agricultura and Direccion General de Flora y Fauna Silvestre/Ministerio de Agricultura (Autorización N0 130-2007-INRENA-IFFS-DCB; N0 122-2008-INRENA-IFFS-DCB; N0 102-2009-AG-DGFFS-DGEFFS and N0 384-2010-AG-DGFFS-DGEFFS; Nº020-2012-AG-DGFFS-DGEFFS; Nº 056-2013-AG-DGFFS-DGEFFS and Nº 058-2013-AG-DGFFS-DGEFFS), IIAP, SPDA, AMPA, PEAH, PEAM and APECO. We also thank the Editor and anonymous reviewers who commented on a previous version of this manuscript, their comments greatly improved this manuscript. Finally we thank the countless local authorities and campesinos for all their help and guidance whilst looking for the monkeys.
Author Details: Sam Shanee is a pimatologist, conservationist and co-founder of Neotropical Primate Conservation. He currently lives in Peru where he co-manages NPC’s research and conservation activities focuseing on the ecology and conservation of Peru’s endemic primates. Nestor Allgas is a biologist from San Marcos University in Lima and founding president of Asociacion Neotropical Primate Conservation - Peru. His main areas of interest are primatology and herpetology. Noga Shanee is co-founder of Neotropical Primate Conservation and co-director of the Yellow-tailed Woolly Monkey project. She specializes tackling illegal wildlife trafficking and her main research interests are the political ecology of biodiversity loss and the connections between cultures, religions and the environment. Nicola Campbell has worked in conservation in Peru with Netoropical Primate Conservation and the CREES Foundation. She has masters degree in Primate Conservation from Oxford Brookes University UK and currently teaches biology at Cirencester College, UK.
Author Contribution: SS was involved in all aspects of this study from survey design, field work, modeling, analysis of results and writing the manuscript. NA was involved in survey design and was the prinicple researcher during field work. NS was involved in survey design, field work and helped analyse results and editing of the final manuscript. NC was involved in survey design and carried out field work in Amazonas and San Martin.
Abstract: Aotus miconax is endemic to Peru and remains one of the least studied of all Neotropical primate taxa. It has an altitudinally restricted distribution and is limited to areas of premontane and montane cloud forest in the countries north. Deforestation in the area is the highest in the country. In many areas deforestation has fragmented remnant populations of A. miconax to isolated forest fragments with high hunting pressure. Our aim was to gather information on the current distribution of A. miconax and other Aotus species in northeastern Peru. Through field surveys we found evidence of the presence of Aotus spp. at 44 localities in the departments of Amazonas, Huánuco, La Libertad and San Martin, including 23 visual observations and four aural detections and from secondary evidence at a further 17 sites. Aotus miconax was found at sites between 1200–3100 m. Combining GIS and maximum entropy ecological niche modelling we predicted the probable original distribution of A. miconax. We also evaluated the current area of occupancy, level of fragmentation and anthropogenic threats faced by this species. The current area of occupancy of A. miconax is much reduced and anthropogenic threats to this species are severe and increasing. The current IUCN Red List status (VU) underestimates actual habitat loss and disturbance. Sympatric species which suffer from similar levels of hunting and habitat loss are considered ‘Critically Endangered’ (IUCN 2011) and based on our estimate of ~60% habitat loss, with much of the remaining habitat highly fragmented; we would like to suggest that A. miconax be classified as Endangered.
Keywords: Aotus nancymaae, Aotus nigriceps, conservation, maximum entropy, Owl Monkey.
Spanish Abstract: Resumen: Aotus miconax es endémico al Perú y una de las especies menos estudiadas de todas las taxas de primates neotropicales. Aotus miconax tiene una distribución restringida altitudinalmente y está limitado a áreas premontanas y montanas de bosque nublado. La deforestación en el área es la más alta en el país. En muchas áreas la deforestación ha dejado que poblaciones de A. miconax estén persistiendo en fragmentos de bosques aislados y enfrentan una alta presión de caza. Nuestro objetivo es reunir información en la actual distribución de A. miconax y otras especies de Aotus en el noreste del Perú. A través de entrevistas de campo encontramos evidencia de la presencia de Aotus spp. en 44 localidades del departamento de Amazonas, Huánuco, La libertad y San Martín, incluyendo 23 observaciones visuales y cuatro detecciones auditivas y por evidencia secundaria por al menos 17 lugares. Aotus miconax estuvo presente entre 1200 y 3100 m.s.n.m. Combinando GIS y un modelo máximo nicho de entropía ecológica predecimos la original distribución de A. miconax. También evaluamos el nivel de fragmentación y la amenaza antropogénica que enfrentan estas especies.Nuestro resultado demuestra que la area de occupancia de A. miconax está reducida y las amenazas antropogénicas son severas y incrementando. El último estado de la RedList (VU) menosprecia la actual perdida de hábitat y perturbación. Especies simpátricas las cuales sufren de un nivel similar de caza y pérdida de hábitat están consideradas En Peligro Crítico y basado en nuestra estimación de ~ 60% perdida de hábitat, con más de la restante alta fragmentación de hábitat; recomendamos que A. miconax este clasificado como En Peligro.
Introduction
The Peruvian Night Monkey Aotus miconax is one of Peru’s eight endemic primate species (Matauschek et al. 2011; Alfaro et al. 2012; Boubli et al. 2012; Wilson et al. 2013; Marsh 2014). Aotus miconax was first described by Thomas (1927a) from specimens collected in Amazonas Department with further specimens collected in Huánuco Department (Hershkovitz 1983). This species remains one of the least known of all primates, with few published field observations (Butchart et al. 1995a,b; Cornejo et al. 2008; Shanee & Shanee 2011; Sanchez-Larranega & Shanee 2012) and only one previous behavioural study (Shanee et al. 2013). Aotus miconax is listed as Endangered on Appendix II of CITES (2005) and as Vulnerable (IUCN category A2c) on the IUCN Red List of Threatened Species.
The distributions of Aotus spp. in northern Peru are poorly understood and no previous distribution survey has been made of A. miconax. This species is largely sympatric with Lagothrix flavicauda (Shanee 2011) and is limited to areas of pre-montane and montane cloud forest in the departments of Amazonas, Huánuco, La Libertad, Loreto and San Martin (Aquino & Encarnacion 1994; Cornejo et al. 2008) and possibly Pasco. Aotus miconax has a marginally wider altitudinal range than L. flavicauda, occurring from just below 1,000m (Thomas 1927b; Cornejo et al. 2008) to over 3,100m. in the Santuario Nacional Cordillera de Colan in Amazonas Department (Campbell 2011). This species has been recorded in Ficus spp. dominated pre-montane and montane cloud forest and white sand forest (Cornejo et al. 2008; Shanee & Shanee 2011; Shanee et al. 2013).
Deforestation in northern Peru is among the highest in the country (Elgegren 2005), fuelled by high immigration rates and the need for agricultural land and timber extraction (Elgegren 2005; Shanee 2011; Shanee 2012a). The widespread deforestation and habitat fragmentation has, in many areas, forced A. miconax into isolated fragments exposing the species to increased parasite load and hunting (Shanee & Shanee 2011; Shanee 2012; Sanchez-Larranega & Shanee 2012; Shanee et al. 2013). There is almost certainly a downward trend in this species population size because of habitat loss.
Geographic Information Systems (GIS) have become an invaluable tool for species distribution modelling (Dunning et al. 1995; Stone et al. 2013). Using data on species presence and absence, combined with information on environmental characteristics, various techniques have been developed for modelling species’ distributions (Guisan et al. 2007; Boubli & Lima 2009; Elith & Graham 2009; Norris et al. 2011). Among these, maximum entropy ecological niche modelling using Maxent program (Phillips et al. 2006) has been shown to perform well compared with other modelling techniques (Elith et al. 2006; Guisan et al. 2007; Elith & Graham 2009; Giovenelli et al. 2010) and has been used in previous studies on a wide range of primate species (Thorn et al. 2008; Boubli & Lima 2009; Willems & Hill 2009; Norris et al. 2011; Peck et al. 2011; Vidal-Garcia & Serio-Silva 2011).
Through field surveys and GIS based distribution modelling we estimated the original distribution of A. miconax and evaluated the current ecological and anthropogenic threat to A. miconax. Specifically, we aimed to gather up-to-date information on the actual distributions of A. miconax and other Aotus spp. in northern Peru. With this data we modelled the predicted original and current extent of A. miconax distribution using maximum entropy algorithm ecological niche modelling with Maxent and ArcGIS programs (Phillips et al. 2006). We extended this using available data on forest cover and proximity away from human settlement and infrastructure to estimate fragmentation and as proxy measures of anthropogenic threat from hunting (Bodmer et al. 1997; Peyton et al. 1998; Michalski & Peres 2005; Buckingham & Shanee 2009; Shanee et al. 2011).
Methods
Study sites
We surveyed sites in the pre-montane and montane cloud forest belt in the eastern branches of the Andean Cordillera in northeastern Peru between 05022’–10001’S & 78026’–75032’W (Fig. 1). We surveyed forested areas at altitudes between 300–3,500 m in the departments of Amazonas, Huánuco, La Libertad and San Martin. During surveys we recorded temperatures between 8–30 0C in the daytime and 3–19 0C at night. Rainfall was variable with monthly averages between ~15mm in the dry season, August to November, and ~1500mm in the wet season, December to May.
Field surveys
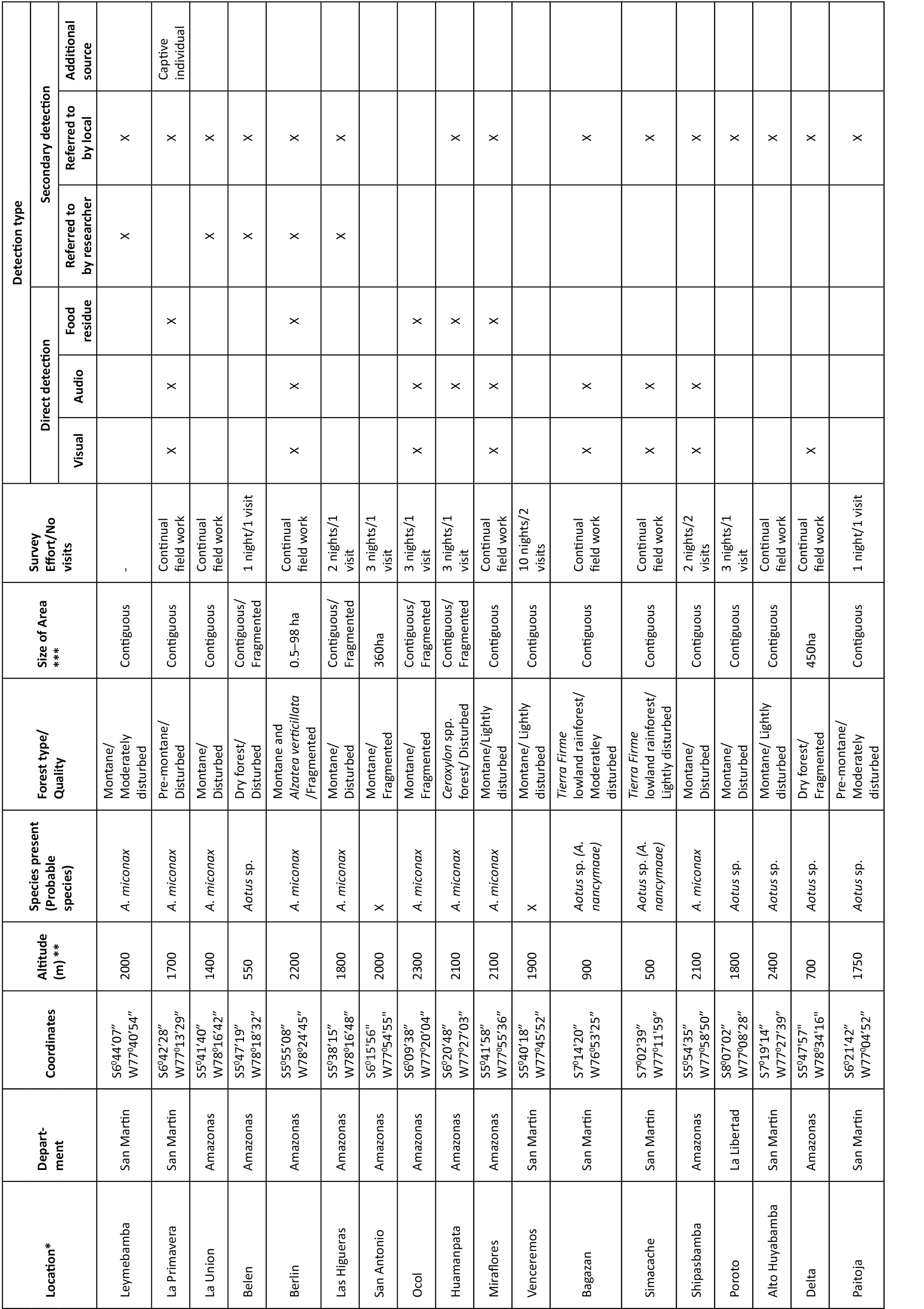
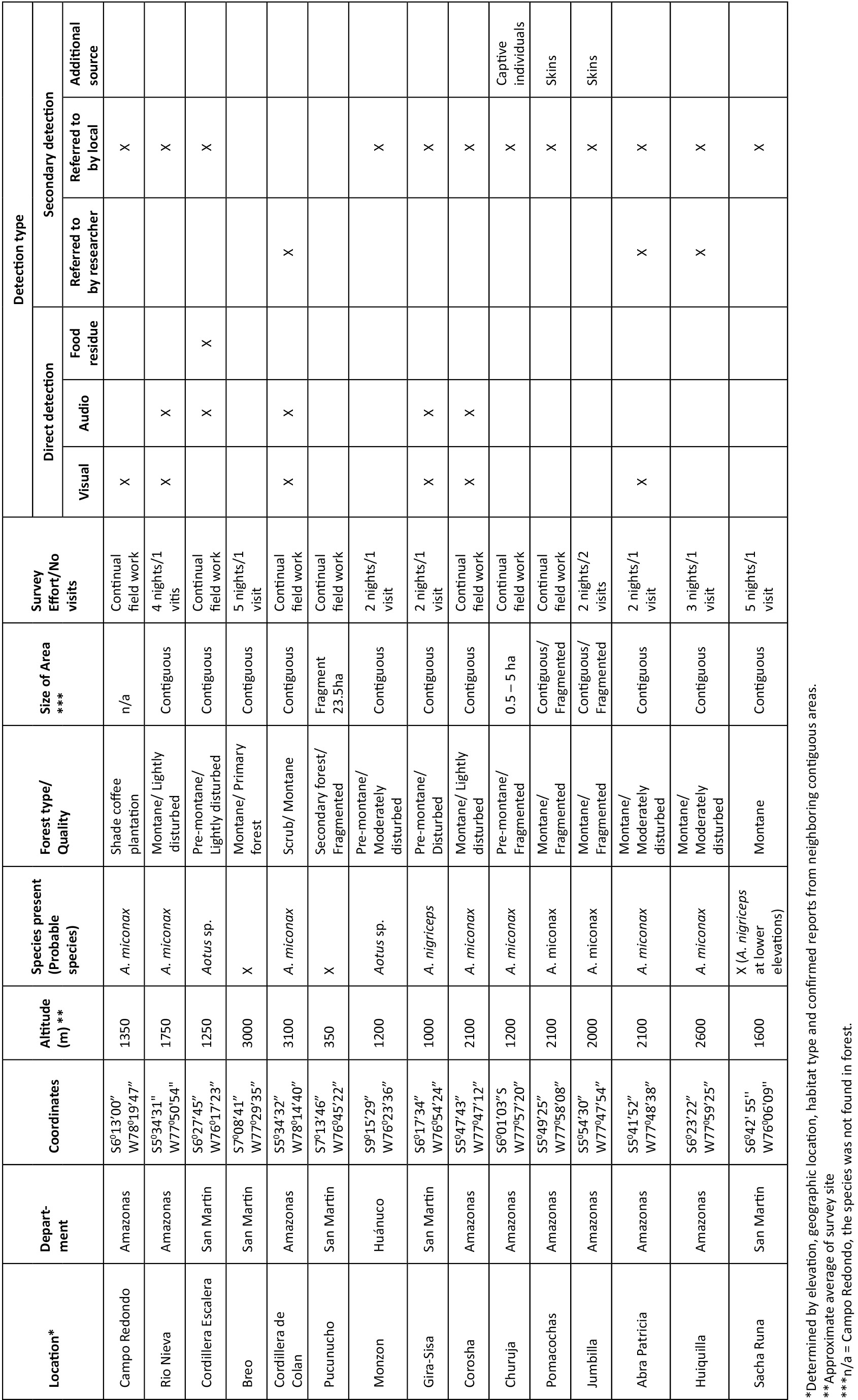
We conducted field surveys between March 2009 and March 2013. We chose survey sites based on records from previous surveys (Butchart et al. 1995a; Cornejo et al. 2008; Shanee 2011) and our preliminary GIS analyses of deforestation. Survey sites included forest fragments, from ~0.5ha to over 50ha and areas of contiguous forest. Some ad hoc observations were also made in gallery forests, stands of individual trees and shade grown crop plantations (Table 1).
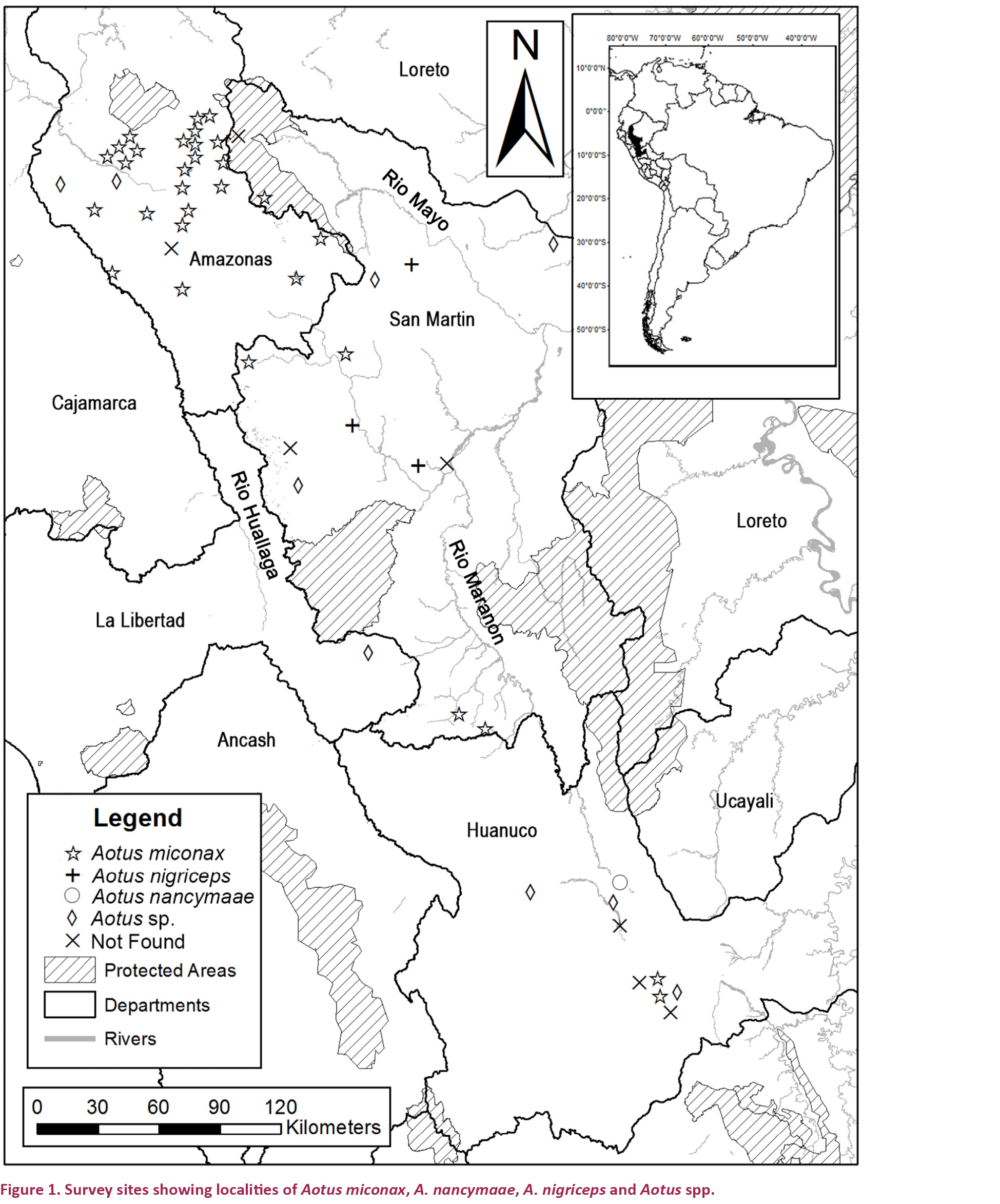
We identified species based on pelage and vocalizations. The three night monkey species we expected to encounter, A. miconax, A. nancymaae and A. nigriceps, belong to the red-necked group (together with A. azarae and A. infulatus) (Hershkovitz 1983; Groves 2001). Although not readily distinguishable, we identified species through direct observation and detailed revision of photographs taken in the field with descriptions given by Groves (2001) and Aquino & Encarnacion (1994). We also compared photographs and accounts given in Rowe & Myers (2012) with our own observations and photographs. Playback of pre-recorded territorial calls (using a portable MP3 player and 1.5 watt speaker) were also used to aid localization and identification of Aotus spp. in areas of sympatry with other large nocturnal mammals. This was particularly useful for A. miconax through comparison with recordings made during behavioural studies at our main field site at La Esperanza, Amazonas Department (Shanee et al. 2013; unpublished data). Species were never identified based solely on vocalisations. When we were not sure of the species identity we report it here as Aotus spp. except in cases for A. miconax where based on elevation, geographic location, habitat type and confirmed reports from neighbouring contiguous areas we tentatively identified to species level (Table 1; Images 1–2).

Sympatric nocturnal species of similar size to Aotus spp. in our area are Potos flavus, Bassaricyon gabbii and Didelphis spp. The latter are easily distinguishable from Aotus spp., P. flavus and B. gabbii are more similar and are often considered primates by local people, particularly because of the prehensile tail of P. flavus. When collecting secondary data from local informants, species identification was made using photographs and drawings and detailed explanations of pelage, size and ecology. Positive identifications were cross-referenced between informants and we asked further details of behaviour, diet and locomotion to ensure identification (Shanee 2011). We recorded presence of Aotus spp. at the generic level from informants, but never inferred species identification based solely on interviews (Shanee 2011). During field visits we interviewed hunters and asked to view captive animals, skins, skulls and miscellaneous body parts of animals hunted in the area. These were used in identification when the locality of the capture could be confirmed. We were always careful not to encourage hunting when asking to see skins, wild caught pets and body parts.
We collected primary data during forest walks along existing trails accompanied by local residents as field guides. Some trails were also made to enter new areas, but this was kept to a minimum to limit forest disturbance (Shanee 2011). We visited field sites during two to seven days, making up to three visits per site (Table 1). The duration of field visits depended on whether or not the presence of Aotus spp. could be confirmed or when secondary evidence showed a high possibility of them being found. Because of the non-stratified sampling effort the time and distance spent walking trails varied depending on site-specific limitations, such as patch size and existing access routes resulting in variable survey effort between sites. The location of all sites was recorded with a handheld GPS (Garmin GPSMap 60CSx), as were points of visual, audio or incidental (e.g., food residues showing clear bite marks) detection. We never inferred the species presence from bite marks without additional evidence. We also collected data on threats to habitat in areas we visited.
Habitat loss analysis
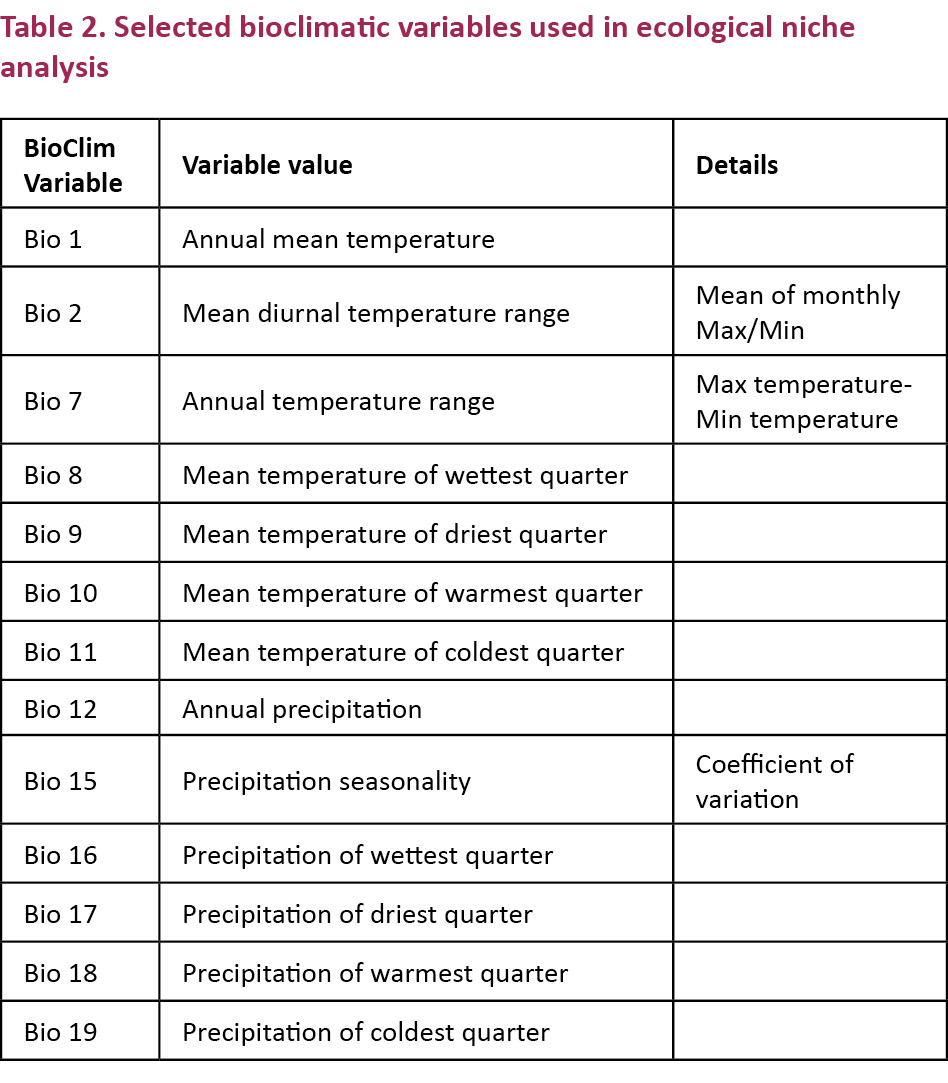
To model the predicted original distribution of A. miconax we used presence-only data in Maxent Program (Phillips et al. 2006; Phillips & Dudík 2008) and ArcGIS 10.1 (ESRI 2012) for analysis and modelling, using 1km resolution environmental layers from BioClim (Hijmans et al. 2005). We selected 13 environmental layers (Table 2) which represent important variables for the presence and maintenance of pre-montane and montane cloud forests and their internal diversity (Webster 1995; Bruijnzeel & Veneklaas 1998; Rapp & Silman 2013). These included bioclimatic variables representative of annual trends, seasonality and limiting factors (Table 2). Also, 30m digital elevation model (DEM) derived from the ASTER (Advanced Spaceborne Thermal Emission and Reflection Radiometer) satellite and a vegetation layer from the terrestrial conservation assessment (Olson et al. 2001). We resampled all layers to ~90m spatial resolution and clipped these to a calibration area of ~700,000km2 that approximated the study area, including most of the Peruvian Andes as well as some lowland Amazonian forest and coastal areas. We used the results of our field studies inputting points of confirmed presence and ran tests using default settings; convergence threshold = 10−5, maximum iterations = 1000, regularization value β = 10−4 and use of linear, quadratic, product and binary features (Phillips et al. 2006).
Taking into account a previous study (Shanee et al. 2013) which reported a home range size for a group of A. miconax at just over 1ha, we used a regularization multiplier of two to better model the species’ use of territory. We set the percentage of test points at 25 and replicate runs using subsamples (Anderson et al. 2002). As ecological niche modelling with Maxent does not take into account geographic boundaries such as major rivers (Gascon et al. 2000), which are particularly important for limiting the distributions of smaller bodied primate species (Ayres & Clutton-Brock 1992), we created a mask based on the Maranon and Huallaga river systems and clipped the Maxent output to within these limits.
Distribution analysis
Canopy cover and vegetation density are important factors for predicting the presence of A. miconax (Campbell 2011). To model the current distribution of Aotus miconax we used a forest cover layer at 30m resolution from (http://earthenginepartners.appspot.com/science-2013-global-forest) (Hansen et al. 2013). As this data is to the year 2000 we combined it with estimates of forest loss and gain to the year 2012 from the same source. Although quantitative data are given by Campbell (2011) we selected a conservative threshold of 50% forest cover for likely presence of the species based on previous studies of primates and definitions of forested/deforested ecosystems (Johnson et al. 2005; Hartley & Hunter 1998) and removed cells with lower values from the map. We then overlaid this onto the Maxent outputs to get an estimate of possible current area of occupancy.
To better model actual and future habitat disturbance and anthropogenic threat level we made additional analysis of proximity away from human settlements and highways as an index of fragmentation and hunting pressure. Using data layers of the national, regional and local road systems as well as cities, towns and villages provided by governmental authorities. Using this final layer meant that only larger villages (those large enough to have schools) were included in the analysis. Also many additional roads are found in the survey area commissioned by local authorities and have not yet been added to the national road systems database, thus we modelled minimum habitat loss and disturbance. We used a crude maximum estimated extent of anthropogenic disturbance as areas ≤5km from human settlement or highways; this represents an intermediate distance used by previous studies (Peyton et al. 1998; Michalski & Peres 2005; Buckingham & Shanee 2009; Shanee et al. 2011). We also calculated a minimum estimated extent of disturbance as ≤1km away from human settlements and highways. We used this smaller distance as previous studies have shown adaptability in A. miconax allowing them to persist close to human settlements in some areas (Shanee & Shanee 2011; Sanchez-Larranega & Shanee 2012; Shanee et al. 2013). All maps were made using three occurrence probability levels (0–19.9 %, 20–49.9 % and >50%) based on values above the minimum threshold given in the Maxent output.
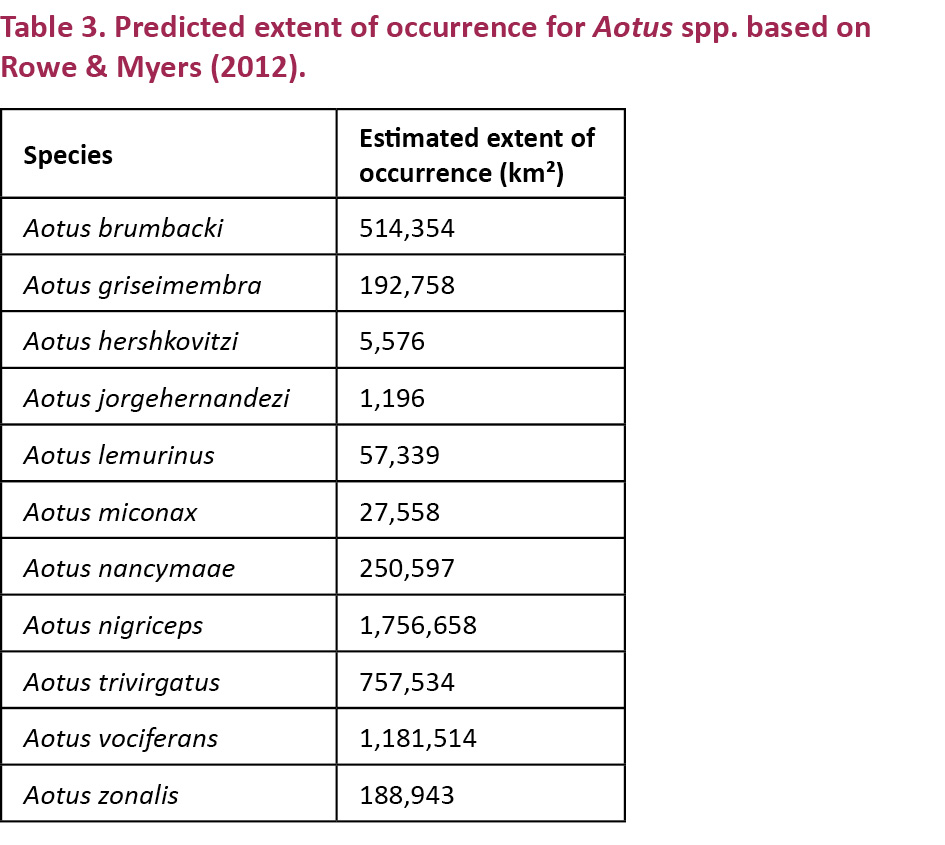
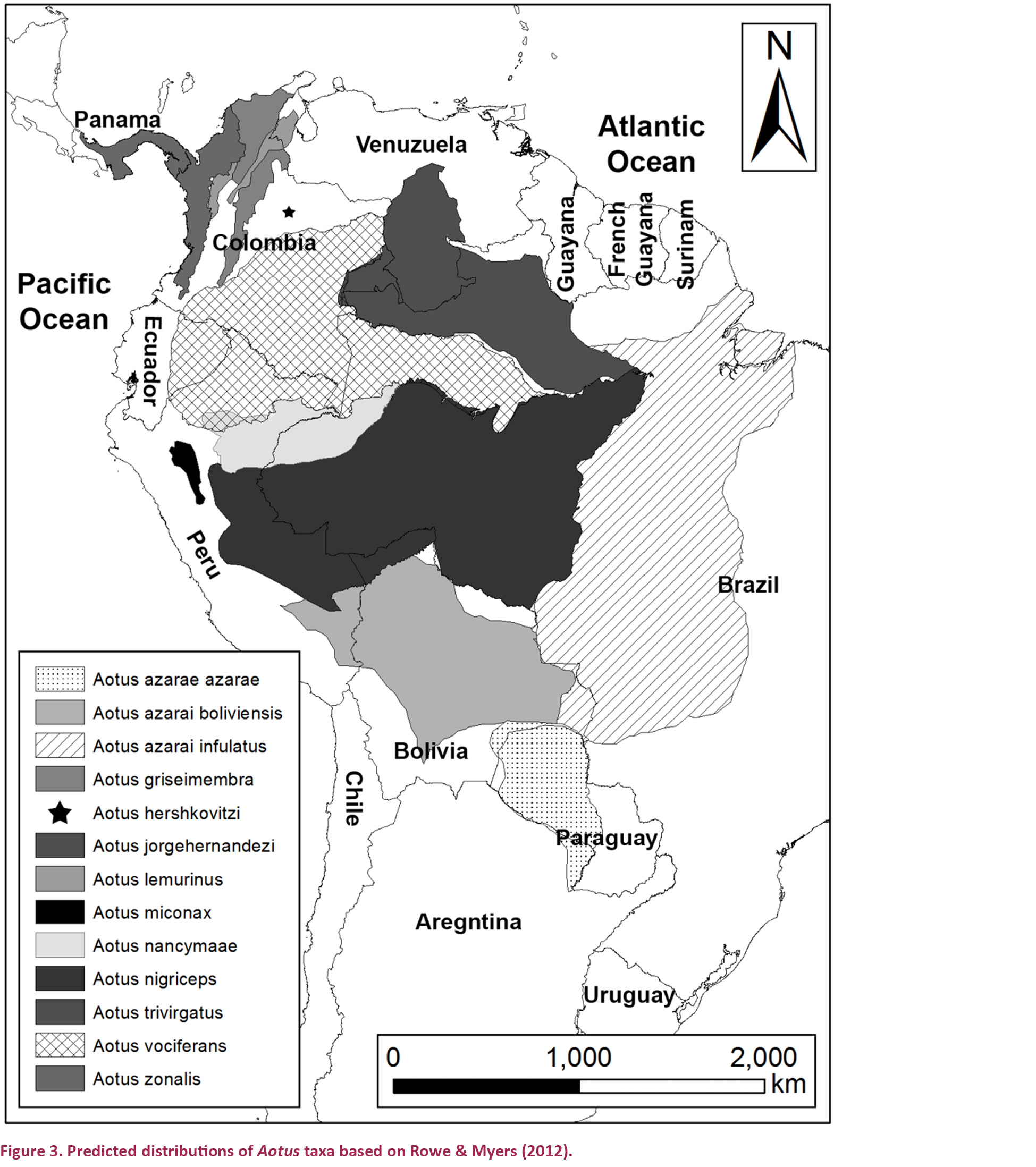
For comparison between the distributions of Aotus spp. we also calculated rough estimates of the distributions of other Owl Monkey species based on freely available shape files (Rowe & Myers 2012) of the estimated distributions.
Fragmentation
To estimate current levels of habitat fragmentation we used ArcGIS to calculate the area of all separate polygon features in the current habitat layer produced from our Maxent outputs. First we removed areas with < 50% forest cover from the Maxent output. To better estimate actual connectivity/fragmentation of Aotus miconax sub-populations we aggregated all polygon features (i.e., forest fragments) > than 1.25ha (Shanee et al. 2013) within a buffer of 200m to single polygons. We chose this threshold as an intermediate distance from observations of travel between forest patches by A. miconax in fragmented habitat (Shanee & Shanee 2011). We then selected three thresholds representing different conservation units for the species: fragments >1.25ha, based on species home range estimate (Shanee et al. 2013); fragments >50ha to represent an estimate of the area needed to support a minimum viable effective population size to retain reproductive fitness (Franklin 1980) based on the >1.25ha home range estimates (Shanee et al. 2013); and areas >10,000ha to represent large areas with contiguous populations as important conservation units. We then overlaid this with our 1km buffer layer of anthropogenic development to highlight areas of high conservation priority.
Results
Field surveys
We surveyed a total of 88 sites during field surveys representing proximately 530 field nights. We combined results from some field sites for analyses because of their close proximity, leaving a total of 52 separate localities (Table 1). We found evidence of the presence of Aotus spp. at 44 localities. We observed wild Night Monkeys 23 times; with an additional four aural encounters. We also found secondary evidence of night monkeys at 44 localities, of these eight were accounts from other researchers active at the site during our surveys and at five sites we found skins or live captive individuals (Table 1). Local informants told us of the presence of Aotus spp. at 42 of the sites that we visited. We only found Night Monkeys (A. miconax) at one site where local informants had not previously confirmed the species presence.
We recorded A. miconax at 31 sites in Amazonas, Huánuco, La Libertad and San Martin (Fig. 1), A. nancymaae was registered once, near Tingo Maria, Huánuco Department (Fig. 1) with another two probable records in San Martin, although we were unable to confirm the species identity. Aotus nigriceps was recorded once in north-central San Martin department (Fig. 1) and another probable record at SachaRuna to the east of the Rio Huallaga in San Martin Department (Fig. 1), again we were not able to confidently identify this record to species level. At a further nine sites we recorded the presence of night monkeys but were unable to determine the species (Fig. 1). At seven sites we found no evidence of night monkeys, neither during fieldwork nor from local informants or other researchers.
All sites where we recorded the presence of A. miconax were between 1200–3100 m. The majority of records for A. miconax were in Ficus spp. dominated pre-montane and montane forests. We also recorded the presence of A. miconax in other forest types, including; white sand forest, Podocarpus spp. dominated cloud forest and Alzatea verticillata dominated forest. At three locations we inferred the presence of A. miconax from secondary evidence but only at sites where neighbouring contiguous forests had confirmed presence (Table 1). At three sites where we expected to find A. miconax populations; Breo, San Antonio and Venceremos (Table 1) we found no evidence of this or other Aotus spp.
Levels of deforestation were high throughout the survey area, even in remote areas such as Breo and Simacache in San Martin and nationally protected areas, such as the Bosque Proteccion Alto Mayo. In some areas, most notably Campo Redondo, Churuja and Delta in Amazonas, almost none of the original forest cover remains. At Campo Redondo we found A. miconax living in shade tree species (Inga edulis) used for coffee (Coffea sp.) plantations (Table 1). Hunting was also found to be a problem for all species of night monkey throughout the area. During surveys we recorded seven captive individuals and numerous skins, skulls and stuffed animals (Table 1).
Predicted distribution limits for A. miconax are the highlands of La Libertad and the Rio Maranon to the west, the lowlands of the Rio Maranon in Amazonas to the north and the lowlands of Loreto to the north-east, the lowlands of the Rio Huallaga valley to the east. The southern limit of this species distribution could not be determined with confidence. The distributional limits for A. nancymaae in this area are the foothills of the Andes above ~1,000m to the west of the Rio Huallaga in Huánuco and San Martin. The distributional limit of A. nigriceps in this area appears to be the Rio Huallaga.
Habitat modelling
The final ecological niche model for Aotus miconax using Maxent Program (Phillips et al. 2006) gave a ROC (Receiver Operating Characteristic) curve AUC (Area Under Curve) of 0.986 for training data and 0.978 for test data. Minimum training presence was 0.053, and its value of statistical significance for presence in the binomial test of omission was 0.067 (p = 6.182 10-39). Results of the jack knife test showed the environmental variable with highest gain (had the most information when used in isolation) when used alone was precipitation of the wettest quarter. The environmental variable that decreased gain the most when omitted (had the most information not represented by the other variables) was the vegetation layer (Olson et al. 2001).
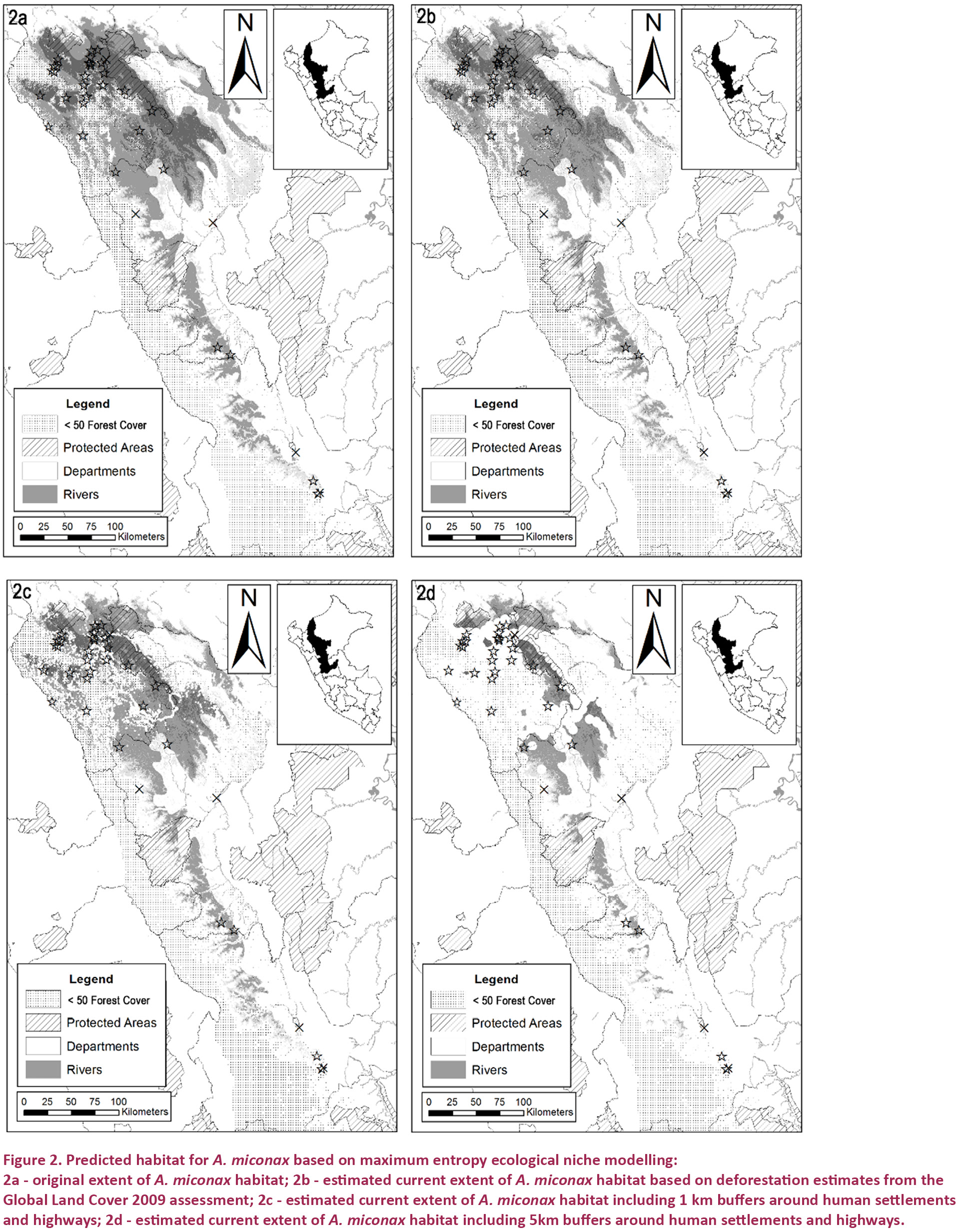
When clipped to within known geographical boundaries, and including all cells with training presence ≥0.1, the total original possible extent of occurrence of A. miconax was estimated to be 32,993km². This area was reclassified into three levels representing low, medium and good probabilities for the presence of A. miconax (0–19.9 %, 20–49.9 % and >50%). Excluding areas of lowest probability the original extent of habitat was estimated to be 25,144km², of which only 6,314km² was in the top category (Fig. 2a). Using data from Hansen et al. (2013) we removed areas with ≤50% forest cover (including estimates of forest loss and gain between 2000–2012), extent of available habitat with >50% forest cover for the three probability levels is 27,237, 20,794 and 5,134 km², giving an average estimate of remaining habitat of 17,721, or 53% (Fig. 2b).
Using a minimum estimate of anthropogenic habitat disturbance, ≤1km away from areas of human settlement and highways, the estimated area of occupancy available for A. miconax, habitat that showed little or no disturbance and where hunting pressure is estimated to be low was 24,854, 18,795 and 4,341km², giving an average estimate, including a lightly disturbed habitat area, of 15,996km², or 48% of the original extent (Fig. 2c). With a maximum estimate of anthropogenic habitat disturbance and hunting, ≤5km away from areas of human settlement and highways, undisturbed habitat remaining for A. miconax for the three probability levels is 10,705, 7,884 and 1,202 km², giving an average estimate of undisturbed habitat of 6,627km², or just 20% of the original extent (Fig. 2d). Averaging the three different estimates of habitat loss/disturbance gives an estimate of 13,448 km², or 40%, of remaining habitat.
Estimates of the area of occurrence of Aotus spp. using existing distribution maps (Rowe & Myers 2012) varied greatly between species. A. jorgehernandezi and A. hershkovitzi were predicted to have had the smallest historical distributions, 1,000 and 5,000 km² respectively. Whilst A. nigriceps and A. vociferans had predicted ranges many times larger (Fig. 3 and Table 3). A. azarai was not included in analysis as its habitat is naturally fragmented and in many areas restricted to gallery forests (Fernandez-Duque et al. 2001) making predictions from available maps very inaccurate.
Fragmentation
Levels of fragmentation of Aotus miconax habitat were extremely high. Our estimate of available habitat included 73,639 fragments, average fragment size was 6.8 ha (min <1, max 62,060ha, ±324.21). Using our three thresholds (>1.25ha, >50ha and >10,000ha) there were 22,590 fragments, average size 20.7ha (min 1.25, max 62,060, ±585), 222 fragments, average size 669.8ha (min 50.36, max 62,060, ±3751) and 7 large areas as possible conservation units >10,000ha, average size 27,907ha (min 11,208, max 62,060, ±17,904) respectively. Our analysis of connectivity (areas of forest separated by ≤ 200m) gave no areas <1.25ha. The total number of fragments was 3,488, of which 3,294 were between 1.25–50 ha in size, average 6.998ha (min 1.25, max 49.9, ±7.91). Using the two remaining thresholds there were 190 fragments, average size 256ha (min 50.5, Max 5,287, ±596.98) and four large areas as possible landscape level conservation units: 180,600, 239,100, 271,600 and one of 2,219,000ha respectively.
Discussion
No previous reliable range estimates or distribution surveys exist for A. miconax. This species’ endemism and specialized habitat preference to mid and high elevation forest results in naturally restricted distribution and an increased risk from anthropogenic and natural extinction pressures (Pimm et al. 1988; Purvis et al. 2000; Laurance et al. 2002; Zeigler et al. 2013). The species original extent of occurrence, estimated here at between 25,144–32,993 km² is larger than predictions for Peru’s other attitudinally restricted endemic primates, the Yellow-tailed Woolly Monkey Lagothrix flavicauda and the San Martin Titi Monkey Callicebus oenanthe (Luna 1987; Hershkovitz 1949–1988 cited in Ayers & Clutton-Brock 1992; Buckingham & Shanee 2009; Shanee et al. 2011), although the methods used to model these distributions was crude (Buckingham & Shanee 2009; Shanee et al. 2011).
The cryptic nature of Aotus, their small size and nocturnal habits, make field surveys particularly difficult (Fernandez-Duque 2011). This and the physical similarity between species also make field identification difficult. We made every effort to correctly identify to species level, comparing published accounts and photographs with our field observations. Whenever possible we triangulated identification between field sightings, vocalizations, revision of skins and captive individuals, interviews and proximity to known localities. We were able to identify the majority of our sightings to the species level. However, we were still unable to identify which species of Aotus spp. were present at nine of the sites we surveyed (Table 1). It is possible that several of these will also be A. miconax (Table 1) although further study is required to confirm this.
We found evidence of Aotus spp. at most survey sites. The use of existing trails and surveying fragmented habitat could have reduced the possibility of encountering animals at the other sites (Shanee 2011). However, small body size and nocturnal habits probably make Aotus spp. less susceptible to anthropogenic disturbance and we were able to find Aotus spp. in many highly disturbed areas and small forest fragments, including coffee plantations. As with previous surveys in this area of Peru (Bóveda-Penalba et al. 2009; Shanee 2011) our choice of survey sites was non-stratified, visiting sites with existing access routes; although some sites were up to two days walk from the nearest road. In most cases the presence of Aotus spp. in disturbed habitat would suggest its presence in neighbouring primary forest areas.
Aotus miconax appears to be able to adapt to anthropogenic habitat disturbance (Shanee & Shanee 2011; Sanchez-Larranega & Shanee 2012; Shanee et al. 2013). During our surveys we found the species in many disturbed and fragmented sites (Table 1). Similarly this species seems to utilize a variety of natural habitat types including; Ficus spp., Podocarpus spp., montane and pre-montane, white sand, palm dominated and Alzatea verticillata dominated forests although how much the species utilizes these areas is unknown. At Breo and Venceremos, two largely undisturbed and protected sites, the probable absence of A. miconax suggests that some undefined habitat characteristic is important in determining this species micro-level distribution.
We also recorded the presence of Aotus nancymaae and A. nigriceps at two sites in San Martin and Huánuco. A. nancymaae was present at the Serpentario in Tingo Maria (Table 1), an area far to the south of its known distribution (Aquino & Encarnacion 1994; Rowe & Myers 2012). It is probable that our records from other low lying areas, <1,000m, of Huánuco are also A. nancymae, as well as records to the west of the Rio Huallaga in San Martin. This extends the known distribution of this species several hundred kilometres to the south along a narrow band of forest between the Rio Huallaga and the Andean foothills. Our record of A. nigriceps at Gera-Sisa is also outside of the species know distribution to the east of the Rio Mayo in northern San Martin (Shanee et al. 2013).
The distributions of Aotus miconax, A. nancymaae and A. nigriceps are probably limited by physical barriers, less defined ecological barriers and competitive exclusion. A. miconax is limited in the west, north and east by the lowlands of the Rio Maranon and Rio Huallaga. We suggest that the Rio Huallaga constitutes the major geographical barrier restricting the distributions of A. nancymaae and A. nigriceps in central San Martin. The southern distribution of A. miconax is less well defined; reductions in ecological niche suitability and competitive exclusion with A. nancymaae and/or A. nigriceps are the most likely barriers although the exact limits of the species distribution are still unknown.
We believe that this study is the first predictive model of the distribution of Aotus miconax. Our model gave good AUC values, the similarly high values for both test and training data suggest that the model is not overfitted and will have good predictive power (Peterson et al. 2007; Merckx et al. 2011). Ecological niche modelling doesn’t consider physical barriers to species distributions. Similarly Maxent has been shown to overestimate distributions, especially with large calibration area (Giovanelli et al. 2010). In our analysis Maxent predicted an ecological niche that included areas as far as northern Ecuador, far outside the species historical distribution (Aquino & Encarnacion 1994). We were able to eliminate this problem by clipping the predicted distribution within known geographical barriers.
The robustness of results from any predictive modelling technique depends largely on the quality and accuracy of data and environmental layers available (Hernandez et al. 2006; Elith & Graham 2009; Giovanelli et al. 2010). Maxent has consistently been shown as one of the most robust ecological modelling algorithms (Elith et al. 2006; Guisen et al. 2007; Elith & Graham 2009; Giovanelli et al. 2010). However, Maxent has been shown to be sensitive to spatial resolution, threshold selection, calibration area, spatial correlation and accuracy of location data (Elith & Graham 2009; Norris et al. 2011; Bean et al. 2012). Even though the spatial resolution we used ~1ha is very detailed, most environmental layers were resampled from lower spatial resolutions reducing accuracy, however this was necessary to include greater resolution on altitudinal data from the ASTER DEM layer. Even so, at this resolution our model should include all but micro-scale gradients in habitat heterogeneity (Elith & Graham 2009). Our minimum predictive threshold, i.e., those areas with ecological conditions where the species presence was confirmed, was very accurate, training threshold <0.1%. Similarly the accuracy of our location data, coming from our multi-year field surveys and not collections or online databases, was extremely high further increasing the robustness of our model (Bean et al. 2012) and the use of a reduced calibration area will also reduce the possibility of erroneous predictions (Giovanellii et al. 2010).
The predicted historical distribution (maximum extent of occurrence) of Aotus miconax we present is one of the smallest of any Aotus species, between 25,144–32,993 km². Accurate distribution maps for other Aotus spp. are not available. Using information given in Rowe & Myers (2012) we estimate that only A. jorgehernandezi and A. hershkovitzi had smaller historical distributions, of 1,000 and 5,000 km² respectively. Most other Aotus spp., such as A. nigriceps and A. vociferans, have distributions that are many times larger (Hershkovitz 1983; Rowe & Myers 2012), although our estimates (Table 3) are very crude and don’t take into account details of habitat type and availibilty. Based on our results A. miconax now has a much-reduced distribution, extent of occurrence and area of occupancy. Including our analysis of fragmentation, the area of contiguous forests that currently support populations of A. miconax are further reduced. Few areas of over 10,000ha, capable of supporting large, sustainable populations, were found further reducing effective population sizes. The area covered by aggregated fragments ≤200m apart was much larger but much of these areas are not suitable as conservation units as they are highly populated.
Deforestation, habitat disturbance and hunting are major threats to all primate species. Aotus spp. are similarly threatened by these anthropogenic pressures (Redford & Robinson 1987; Shanee 2012b). Levels of deforestation and habitat disturbance in our area were high, with all sites showing at least low levels of disturbance or hunting (Table 1). Our evaluation of habitat loss show that A. miconax should be classified as Endangered on the IUCN Red List (IUCN 2001). Our estimate of only 53% of total original habitat remaining for the species is based on data that do not give enough detail on fragmentation, with some areas classified as forest but are actually heavily fragmented (Sam Shanee pers. obs.). By including extra data on proximity to human settlement, as a measure of fragmentation, estimates of remaining habitat is further reduced. Our 5km buffer is not a good estimate of fragmentation or habitat loss alone, but can be accurate for estimating areas of high hunting pressure (Peyton et al. 1998; Peres 2001;) and is useful for prediciting hotspots of future habitat loss. Conversely, the 1km buffer probably underestimates the effects of proximity to human settlement on forests in all but the least densely populated areas. An intermediate distance would be more accurate in representing actual fragmentation and hunting, resulting in habitat loss over the 50% required by the IUCN Red List categories (IUCN 2001). This will still be an underestimate as our data on human settlement, from the Peruvian Ministry of Education, only includes villages with schools, indicating that the actual number of human settlements, and therefore habitat loss, will be greater. To date the majority of habitat loss has been in peripheral areas of A. miconax distribution. During this and previous surveys (Shanee 2011) we found many new roads under construction, some of which are now completed. This opens new areas of forest to logging, hunting and settlement, which will accelerate future habitat loss. There are several protected areas within the range of A. miconax, although only small portions of these are suitable habitat for this species. Also, many of these suffer from the same problems as surrounding unprotected areas (INRENA 2008; Shanee 2011; Shanee 2012a).
As in previous studies, we found that A. miconax has shown adaptability to anthropogenic habitat disturbance (Cornejo et al. 2008; Shanee & Shanee 2011; Sanchez-Larranega & Shanee 2012; Shanee et al. 2013). This was also true for A. nancymaae and A. nigriceps which we found persisting in disturbed and fragmented areas (Table 1), although we suggest that this is probably true for areas of low hunting pressure only. Hunting has been known to cause localized extinctions of neotropical primate species in fragmented areas, and even in large areas of contiguous forest (Redford & Robinson 1987; Bodmer et al. 1997; Peres & Dolman 2000; Peres 2001; Michalski & Peres 2005). Nocturnal habits, small body size and sub-caudal scent glands make Aotus spp. less desirable, and therefore less susceptible, to hunting than larger bodied diurnal primates (Noga Shanee pers. obs.). Even so, we found many cases of hunting of A. miconax, as have previous studies of hunting in the area (Shanee 2012b). Hunting of Aotus spp. is likely to increase as populations of more desirable species are reduced in parallel with increasing human populations and expansion of the agricultural frontier (Peres 2001; Remis & Robinson 2012).
Until recently the regions where Aotus miconax populations were found remained largely unsettled because of their natural inaccessibility and socio-political unrest (Shanee 2011; Shanee 2012a) from Maoist guerrilla groups, and coca (Erythroxylum coca) cultivation (Young 1996; Schjellerup 2000; Shanee 2012a). Even in these areas habitat destruction is now a major threat. Immigration has led to the clearance of many more accessible areas and the expansion of mining and large scale monocultures mean this immigration is now reaching higher into the Andean foothills (Noga Shanee pers. obs.). The patterns of human development and settlement have, as in many areas (Wade et al. 2003), led to the fragmentation of remaining A. miconax habitat.
Our results provide much needed information on the distribution of Aotus spp. in northeastern Peru and an evaluation of the actual conservation status of A. miconax. The current Red List status (Vulnerable A2c IUCN 2012) underestimates actual habitat loss and disturbance. The sympatric Lagothrix flavicauda is listed as Critically Endangered (CRA4c) under the same categories and has been considered one of the World’s Top 25 Most Endangered Primate Species several times (Mittermeier et al. 2012). Similarly, Callicebus oenanthe, which suffers from similar levels of hunting and habitat loss (Shanee et al. 2011; Shanee 2012b) as A. miconax is considered CR (IUCN 2011) and as one of the Worlds Top 25 Most Endangered Primate Species for the second time (Schwitzer et al. 2014). Our estimate of up to 62% habitat loss, with much of the remaining habitat highly fragmented and hunted shows that A. miconax should be categorized as Endangered under criteria A2ac+A3c+4ac of the IUCN Red List categories (IUCN 2011) based on a decline in area of occupancy.
Further field studies are needed to determine the southern extent of the distribution of Aotus miconax. Also, which habitat characteristics determine the micro-level distributions of Aotus spp., as well as studies on ecology, population densities and genetic variability. As with all models, ours was limited by the quality of data available. However, we feel it is largely accurate and provides important information from which to base subsequent surveys and conservation actions. With developments in modelling additional modelling algorithms could be used with finer resolution geographical data when available. Combining this with additional presence and, if possible, absence data would produce more robust models and the use of additional algorithms could increase confidence in predictions. We also highlight the need for further work in karyotyping the various Peruvian Aotus spp. which would greatly aid in identification, allowing for better knowledge of diversity and distributions.
References
Alfaro, J.W.L., J.D.E.S.E. Silva & A.B. Rylands (2012). How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. American Journal of Primatology 74(4): 273–286; http://dx.doi.org/10.1002/ajp.22007
Alfaro, J.W.L., J.P. Boubli, L.E. Olson, A. Di Fiore, B. Wilson, G.A. Gutiérrez-Espeleta, K.L. Chiou, M. Schulte, S. Neitzel, V. Ross, D. Schwochow, M.T.T. Nguyen, I. Farias, C.H. Janson & M.E. Alfaro (2012). Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. Journal of Biogeography 39(2): 272–288; http://dx.doi.org/10.1111/j.1365-2699.2011.02609.x
Anderson, R.P., M. Gomez-Laverde & A.T. Peterson (2002).Geographic distributions of spiny pocket mice in South America: insights from predictive models. Global Ecology and Biogeography 11: 131–141; http://dx.doi.org/10.1046/j.1466-822X.2002.00275.x
Aquino, R. & F. Encarnacion (1994). Los Primates del Peru. Primate Report 40: 1–130.
Ayres, J.M. & T.H. Clutton-Brock (1992). River boundaries and species range size in Amazonian primates. The American Naturalist 140(3): 531–537; http://dx.doi.org/10.2307/2462782
Bean, W.T., R. Stafford & J.S. Brashares (2012). The effect of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 35: 250–258; http://dx.doi.org/10.1111/j.1600-0587.2011.06545.x
Bodmer, R.E., J.F. Eisenberg & K.H. Redford (1997). Hunting and the likelihood of extinction of Amazonian mammals. Conservation Biology 11(2): 460–466; http://dx.doi.org/10.1046/j.1523-1739.1997. 96022.x
Boubli, J.P. & M.G. Lima (2009). Modeling the geographical Distribution and fundamental niches of Cacajao spp. and Chiropotes israelita in northwestern Amazonia via a maximum entropy algorithm. International Journal of Primatology 30(2): 217–228; http://dx.doi.org/10.1007/s10764-009-9335-4
Boubli, J.P., A.B. Rylands, I.P. Farias, M.E. Alfaro & J.L. Alfaro (2012). Cebus Phylogenetic Relationships: A Preliminary Reassessment of the Diversity of the Untufted Capuchin Monkeys. American Journal of Primatology 74(4): 381–393; http://dx.doi.org/10.1002/ajp.21998
Bóveda-Penalba, A., J. Vermeer, F. Rodrigo & F. Guerra-Vásquez (2009). Preliminary report on the distribution of (Callicebus oenanthe) on the eastern feet of the Andes. International Journal of Primatology 30(3): 467–480; http://dx.doi.org/10.1007/s10764-009-9353-2
Bruijnzeel, L.A. & E.J. Veneklaas (1998). Climatic conditions and tropical montane forest production: The fog has not lifted yet. Ecology 79(1): 3–9; http://dx.doi.org/10.1890/0012-9658(1998)079[0003:ccatmf]2.0.co;2
Buckingham, F. & S. Shanee (2009). Conservation priorities for the Peruvian yellow-tailed Woolly Monkey (Oreonax flavicauda): A GIS risk assessment and gap analysis. Primate Conservation 24(1): 65–71.
Butchart, S.H.M., R. Barnes, C.W.M. Davies, M. Fernandez & N. Seddon (1995a). Observations of two threatened primates in the Peruvian Andes. Primate Conservation 19(1): 15–19.
Butchart, S.H.M., R. Barnes, C.W.M. Davies, M. Fernandez & N. Seddon (1995b). Threatened mammals of the Cordillera de Colán, Peru. Oryx 29(4): 275–281; http://dx.doi.org/10.1017/S003060530002127X
Campbell, N. (2011). The Peruvian night monkey, Aotus miconax; A comparative study of occupancy between Cabeza del Toro and Cordillera de Colan, Peru. MSc Thesis. Department of Social Sciences and Law, Oxford Brookes University, vi+70pp.
Cornejo, F.M., R. Aquino & C. Jimenez (2008). Notes on the natural history, distribution and conservation status of the Andean night monkey, Aotus miconax Thomas, 1927. Primate Conservation 23(1): 1–4.
Dunning, J.B.J., D.S. Stewart, B.J. Danielson, B.R. Noon, T.L. Root, R.H. Lamberson & E.E. Stevens (1995). Spatially Explicit Population Models: Current Forms and Future Uses. Ecological Applications 5(1): 3–11; http://dx.doi.org/10.2307/1942045
Elgegren, J.J. (2005). La deforestación en el Perú. http://www.conam.gob.pe/documentos/Taller-analisis-Ambiental/La_Deforestacion_en_el_Peru.pdf Electronic version accessed 1 August 2008
Elith, J. & C.H. Graham (2009). Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 32: 66–77; http://dx.doi.org/10.1111/j.1600-0587.2008.05505.x
Elith, J., C.H. Graham, M. Dudik, S. Ferrier, A. Guisan, R.J. Hijmans, F. Huettmann, J.R. Leathwick, A. Lehmann, J. Li, L.G. Lohmann, B.A. Loiselle, G. Manion, C. Moritz, M. Nakamura, Y. Nakazawa, J.M.M. Overton, A.T. Peterson, S.J. Phillips, K. Richardson, R. Scachetti-Pereira, R.E. Schapire, J. Soberon, S. Williams, M.S. Wisz & N.E. Zimmermann (2006). Novel methods improve prediction of species’distributions from occurence data. Ecography 29: 129–151; http://dx.doi.org/10.1111/j.2006.0906-7590.04596.x
ESRI. (2012). ArcGIS Desktop (Version 9.3). New York: Environmental Systems Research Institute.
Fernandez-Duque, E. (2011). Aotinae: Social monogamy in the only nocturnal haplorhines, pp. 139–154. In: Campbell, C.J., A. Fuentes, K.C. Mackinnon, M. Panger & S.K. Bearder (eds.). Primates in Perspective. Oxford University Press, UK, 720pp.
Fernandez-Duque, E., M. Rotundo & C. Sloan (2001). Density and population structure of Owl Monkeys (Aotus azarai) in the Argentinian Chaco. American Journal of Primatology 53: 99–108; http://dx.doi.org/10.1002/1098-2345(200103)
Franklin, I.R. (1980). Evolutionary change in small populations, pp. 134–150. In: Soule M.E. & B.A. Wilcox (eds.). Conservation Biology: an Evolutionary-Ecological Perspective. Sinauer Associates, Sunderland, 395pp.
Gascon, C., J.R. Malcolm, J.L. Patton, M.N.F. da Silva, J.P. Bogart, S.C. Lougheed, C.A. Peres, S. Neckel & P.T. Boag (2000). Riverine barriers and the geographic distribution of Amazonian species. Proceedings of the National Academy of Sciences 97(25): 13672–13677; http://dx.doi.org/10.1073/pnas.230136397
Giovanellii, J.G.R., M.F. de Siqueira, C.F.B. Haddad & J. Alexandrino (2010). Modelling a spatially restricted distribution in the Neotropics: How the size of calibration area affects the performance of five presence-only methods. Ecological Modelling 221: 215–224; http://dx.doi.org/10.1016/j.ecolmodel.2009.10.009
Groves, C.P. (2001). Primate Taxonomy. Smithsonian Institution Press, New York, 350pp.
Guisan, A., N.E. Zimmermann, J. Elith, C.H. Graham, S. Phillips & A.T. Peterson (2007). What matters for predicting the occurrences of trees: Techniques, data, or species’characteristics? Ecological Monographs 77(4): 615–630; http://dx.doi.org/10.1890/06-1060.1
Hansen, M.C., P.V. Potapov, R. Moore, M. Hancher, S.A. Turubanova, A. Tyukavina, D. Thau, S.V. Stehman, S.J. Goetz, T.R. Loveland, A. Kommareddy, A. Egorov, L. Chini, C.O. Justice & J.R.G. Townshend (2013). High resolution global maps of 21st-century forest cover change. Science 342(6160): 850–853; http://10.1126/science.1244693
Hartley, M.J. & M.L. Hunter (1998). A meta-analysis of forest cover, edge effects, and artificial nest predation rates. Conservation Biology 12(2): 465–469; http://10.1111/j.1523-1739.1998.96373.x
Hernandez, P.A., C.H. Graham, L.L. Master & D.L. Albert (2006). The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29: 773–785; http://dx.doi.org/10.1111/j.0906-7590.2006.04700.x
Hershkovitz, P. (1983). Two new species of night monkeys, genus Aotus (Cebidae, platyrrhini): A preliminary report on Aotus taxonomy. American Journal of Primatology 4(3): 209–243; http://dx.doi.org/10.1002/ajp.1350040302
Hijmans, R.J., S.E. Cameron, J.L. Parra, P.G. Jones & A. Jarvis (2005). Very high resolution interpolated climate surfaces for global land areas. Internationl Journal of Climatology 25: 1965–1978; http://dx.doi.org/10.1002/joc.1276
INRENA (2008). Bosque Proteccion Alto Mayo: Plan maestro 2008-2013. http://www.gtz-rural.org.pe/?apc=Pa-ijm-t-w1–andx=1069. Electronic version accessed 01 March 2009
IUCN (2001). IUCN Red List Catagories and Criteria: Version 3.1. In: IUCN 2013. 2013 IUCN Red List of Threatened Species. Downloaded 01 March 2013.
IUCN (2011). Callicebus oenanthe. In: IUCN 2013. 2013 IUCN Red List of Threatened Species. Downloaded 01 March 2013.
IUCN (2012). Aotus miconx. In: IUCN 2013. 2013 IUCN Red List of Threatened Species. Downloaded 01 March 2013.
Johnson, A., S. Singh, M. Duangdala & M. Hedemark (2005). The Western Black-crested Gibbon Nomascus concolor in Laos: new records and conservation status. Oryx 39(3): 311–317; http://dx.doi.org/10.1017/S0030605305000906
Laurance, W.F., A.K.M. Albernaz, G. Schroth, P.M. Fearnside, S. Bergen, E.M. Venticinque & C. Da Costa (2002). Predictors of deforestation in the Brazilian Amazon. Journal of Biogeography 29(5–6): 737–748; http://dx.doi.org/10.1046/j.1365-2699.2002.00721.x
Luna, M.L. (1987). Primate conservation in Peru: A case study of the Yellow-tailed Woolly Monkey. Primate Conservation 8(1): 122–123.
Marsh, L.K. (2014). A taxonomic revision of the Saki Monkeys, Pithecia Desmarest, 1804. Neotropical Primates 21(1): 1–163.
Matauschek, C., C. Roos & E.W. Heymann (2011). Mitochondrial phylogeny of tamarins (Saguinus, Hoffmannsegg 1807) with taxonomic and biogeographic implications for the S. nigricollis species group. American Journal of Physical Anthropology 144(4): 564–574; http://dx.doi.org/10.1002/ajpa.21445
Merckx, B., M. Steyaert, A. Vanreusel, M. Vincx & J. Vananerbeke (2011). Null models reveal preferential sampling, spatial autocorrelation and overfitting in habitat suitability modelling. Ecological Modelling 222(3): 588–597; http://dx.doi.org/10.1016/j.ecolmodel.2010.11.016
Michalski, F. & C.A. Peres (2005). Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biological Conservation 124(3): 383–396; http://dx.doi.org/10.1016/j.biocon.2005.01.045
Mittermeier, R.A., C. Schwitzer, A.B. Rylands, L.A. Taylor, F. Chiozza, E.A. Williamson & J. Wallis (2012). Primates in Peril: The worlds 25 most endangered primates 2012–2014. IUCN/SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI) & Bristol Conservation and Science Foundation, Bristol, UK, 40pp.
Norris, D., F. Rocha-Mendes, R. Marques, R.A. Nobre & M. Galetti (2011). Density and Spatial Distribution of Buffy-tufted-ear Marmosets (Callithrix aurita) in a Continuous Atlantic Forest. International Journal of Primatology 32(4): 811–829; http://dx.doi.org/10.1007/s10764-011-9503-1
Olson, D.M., E. Dinerstein, E.D. Wikramanayake, N.D. Burgess, G.V.N. Powell, E.C. Underwood, J.A. D’amico, I. Itoua, H.E. Starnd, J.C. Morrison, C.J. Loucks, T.F. Allnutt, T.H. Ricketts, Y. Kura, J.F. Lamoreux, W.W. Wettengel, P. Hedao & K.R. Kassem (2001). Terrestrial ecoregions of the world: a new map of life on earth. BioScience 51(11): 933–938; http://dx.doi.org/10.1641/0006-3568(2001)051[0933:teotwa]2.0.co;2
Peck, M., J. Thorn, A. Mariscal, A. Baird, D. Tirira & D. Kniveton (2011). Focusing conservation efforts for the Critically Endangered Brown-headed Spider Monkey (Ateles fusciceps) using remote sensing, modeling, and playback survey methods. International Journal of Primatology 32(1): 134–148; http://dx.doi.org/10.1007/s10764-010-9445-z
Peres, C.A. (2001). Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conservation Biology 15(6): 1490–1505; http://dx.doi.org/10.1046/j.1523-1739.2001.01089.x
Peres, C.A. & P.M. Dolman (2000). Density compensation in neotropical primate communities: Evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122(2): 175–189; http://dx.doi.org/10.1007/pl00008845
Peterson, A.T., M. Papeş & M. Eaton (2007). Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography 30(4):550-560; http://dx.doi.org/10.1111/j.0906-7590.2007.05102.x
Peyton, B., E. Yerena, D.I. Rumiz, J. Jorgenson & J. Orejuela (1998). Status of wild Andean bears and policies for their managment. Ursus 10: 87–100.
Phillips, S.J., R.P. Anderson & R.E. Schapire (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling 190(3–4): 231–259; http://dx.doi.org/10.1016/j.ecolmodel.2005.03.026
Phillips, S.J. & M. Dudík (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31(2): 161–175; http://dx.doi.org/10.1111/j.0906-7590.2008.5203.x
Pimm, S.L., H.L. Jones & J. Diamond (1988). On the risk of extinction. The American Naturalist 132(6): 757–785.
Purvis, A., J.L. Gittleman, G. Cowlishaw & G.M. Mace (2000). Predicting extinction risk in declining species. Proceedings of the Royal Society of London. Series B: Biological Sciences 267(1456): 1947–1952; http://dx.doi.org/10.1098/rspb.2000.1234
Rapp, J.M. & M.R. Silman (2013). Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Climate Research 55(1): 17–32.
Redford, K.H. & J.G. Robinson (1987). The game of choice: Patterns of Indian and colonist hunting in the neotropicss. American Anthropologist 89(3): 650–667; http://dx.doi.org/10.1525/aa.1987.89.3.02a00070
Remis, M.J. & C.A.J. Robinson (2012). Reductions in primate abundance and diversity in a Multiuse protected area: synergistic impacts of hunting and Logging in a Congo Basin Forest. American Journal of Primatology 74(7): 602–612; http://dx.doi.org/10.1002/ajp.22012
Rowe, N., & M. Myers (2012). All the worlds primates. http://www.alltheworldsprimates.org. Electronic version accessed 28 May 2012.
Sanchez-Larranega, J. & S. Shanee (2012). Parásitos gastrointestinales en el mono choro cola amarilla (Oreonax flavicauda) y el mono nocturno Andino (Aotus miconax) en Amazonas, Perú. Neotropical Primates 19(1): 38–41.
Schjellerup, I. (2000). La Morada. A case study on the impact of human pressure on the environment in the Ceja de Selva, northeastern Peru. AMBIO: A Journal of the Human Environment 29: 451–454.
Schwitzer, C., R.A. Mittermeier, A.B. Rylands, L.A. Taylor, F. Chiozza, E.A. Williamson, J. Wallis & F.E. Clark (2014). Primates in Peril: The World’s 25 Most Endangered Primates 2012–2014. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Bristol, UK, 87pp.
Shanee, N. (2012a). The dynamics of threats and conservation efforts for the tropical Andes hotspot in Amazonas and San Martin, Peru. PhD Thesis, DICE, Kent University, vii+426pp.
Shanee, N. (2012b). Trends in local wildlife hunting, trade and control in the Tropical Andes Hotspot, northeastern Peru. Endangered Species Research 19(2): 177–186.
Shanee, S. (2011). Distribution survey and threat assessment of the Yellow-tailed Woolly Monkey (Oreonax flavicauda; Humboldt, 1812), Northeastern Peru. International Journal of Primatology 32(3): 691–707; http://dx.doi.org/10.1007/s10764-011-9495-x
Shanee, S., N. Allgas & N. Shanee (2013). Preliminary observations on the behavior and ecology of the Peruvian night monkey (Aotus miconax: Primates) in a remnant cloud forest patch, north eastern Peru. Tropical Conservation Science 6(1): 138–148;
Shanee, S. & N. Shanee (2011). Observations of terrestrial behavior in the Peruvian night monkey (Aotus miconax) in an anthropogenic landscape, La Esperanza, Peru. Neotropical Primates 18(2): 55–58.
Shanee, S., N. Shanee & N. Allgas-Marchena (2013). Primate surveys in the Maranon-Huallaga landscape, northern Peru with notes on conservation. Primate Conservation 27: 3–11.
Shanee, S., J.C. Tello-Alvarado, J. Vermeer & A.J. Boveda-Penalba (2011). GIS risk assessment and GAP analysis for the Andean Titi Monkey (Callicebus oenanthe). Primate Conservation 26(1): 17–23.
Stone, O.L., S. Laffan, D. Curnoe & A.R. Herries (2013). The spatial distribution of Chacma Baboon (Papio ursinus) habitat based on an environmental envelope model. International Journal of Primatology 34(2): 407–422; http://dx.doi.org/10.1007/s10764-013-9669-9
Thomas, O. (1927a). The Godman-Thomas expedition to Peru. On mammals collected by Mr R.W. Hendee in the province of San Martin, N. Peru, mostly at Yurac Yacu. Annals and Magazine of Natural History 9(20): 594–608.
Thomas, O. (1927b). The Godman-Thomas expedition to Peru. On mammals from the Upper Huallaga and neighbouring highlands. Annals and Magazine of Natural History 9(20): 594–608.
Thorn, J., V. Nijman, D. Smith & K.A.I. Nekaris (2008). Ecological niche modelling as a technique for assessing threats and setting conservation priorities for Asian Slow Lorises (Primates: Nycticebus). Diversity and Distributions 15(2): 289–298.
Vidal-Garcia, F. & J.C. Serio-Silva (2011). Potential distribution of Mexican primates: modelling the ecological niche with the maximum entropy algorithm. Primates 52: 261–270; http://dx.doi.org/10.1007/s10329-011-0246-6
Wade, T.G., K.H. Riitters, J.D. Wickham & K.B. Jones (2003). Distribution and causes of global forest fragmentation. Conservation Ecology 7(2): 7.
Webster, G.L. (1995). The panorama of neotropical cloud forests, pp. 53–77. In: Churchill, S.P., H. Balslev, E. Forero & J.L. Luteyn (eds.), Biodiversity and conservation of Neotropical Montane Forests. New York Botanical garden, New York.
Wilson, D.E., R.A. Mittermeier, S. Ruff, A. Martinez-Vilalta & T. Llobet (2013). Handbook of the Mammals of the World: Primates. Lynx Ediciones, Spain, 952pp.
Willems, E.P. & R.A. Hill (2009). A critical assessment of two species distribution models: a case study of the Vervet Monkey (Cercopithecus aethiops). Journal of Biogeography 36(12): 2300–2312; http://dx.doi.org/10.1111/j.1365-2699.2009.02166.x
Young, K.R. (1996). Threats to biological diversity caused by coca/cocaine deforestation in Peru. Environmental Conservation 23(1): 7–15; http://dx.doi.org/10.1017/S0376892900038200
Zeigler, S.L., K.M. De Vieeschouwer & B.E. Raboy (2013). Assessing extinction risk in small metapopulations of Golden-headed Lion Tamarins (Leontipithecus chrysomelas) in Bahia state, Brazil. Biotropica 45(4): 528–535; http://dx.doi.org/10.1111/btp.12037
Table 1. Records from survey sites, species identification and detection type as well as habitat characteristics.