Avian diversity and density estimation of birds of the Indian Institute of Forest Management Campus, Bhopal, India
Anjali Aggarwal 1, Govind Tiwari 2 & Sprih Harsh 3
1 Consultant, Gujarat Tourism Opportunity Limited, Haveli Arcade, Sector - 11, Gandhinagar, Gujarat 382011, India
Present address: Aasha Villa, Gayatri Park, Kalawad Road, Rajkot, Gujarat 360001, India
2 Project Officer, Foundation for Ecological Security, A-1 Madhuram Park, Near Srinathji Society, Ganesh Crossing, Anand, Gujarat 388001, India
Present address: 2548 - ‘Om Bhavan’, 4th Crossing, Chandpole Bazaar, Jaipur, Rajasthan 302001, India
3 Senior Project Officer, WWF-India, House No. 30, Datt Garden View, Near Tilhari Sports Club, Jabalpur, Madhya Pradesh 482021, India
1 anjali.aggarwal191989@gmail.com, 2 govindtiwari007@yahoo.co.in, 3 sprih.harsh@gmail.com (corresponding author)
doi: http://dx.doi.org/10.11609/JoTT.o3888.6891-902 | ZooBank: urn:lsid:zoobank.org:pub:E4384F6F-B4D7-46AB-B1E4-A7B8E93CD9B2
Editor: Rajiv Kalsi, M.L.N. College, Yamuna Nagar, Haryana, India. Date of publication: 26 February 2015 (online & print)
Manuscript details: Ms # o3888 | Received 25 December 2013 | Final received 17 December 2014 | Finally accepted 13 February 2015
Citation: Aggarwal, A., G. Tiwari & S. Harsh (2015). Avian diversity and density estimation of birds of the Indian Institute of Forest Management Campus, Bhopal, India. Journal of Threatened Taxa 7(2): 6891–6902; http://dx.doi.org/10.11609/JoTT.o3888.6891-902
Copyright: © Aggarwal et al. 2015. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
Funding: None.
Competing Interest: The authors declare no competing interests.
Author Details: Author Details: Sprih Harsh is currently working in Satpuda Maikal Landscape, Tiger Conservation Programme (WWF-India). She is looking after conservation of corridors in the landscape for providing a secure dispersal to tiger, co-predators and prey species between different tiger reserves. Anjali Aggarwal worked for Gujarat Tourism Opportunity Limited and Nature Conservation Foundation as a research affiliate in Pakke Tiger Reserve, Arunachal Pradesh on a study to understand factors affecting hornbill breeding success during the breeding season. Govind Tiwari worked with FES and involved in various development and conservation oriented projects in southern Rajasthan. He has also been involved in a study to understand the ecology of Sloth Bear in southern Rajashtan near Phulwari and Kumbhalgarh Wildlife sanctuaries.
Author Contribution: SH contributed in carrying out the study in the field and in the analysis part and played a major role in documenting and preparing the research paper. AA contributed in analysis part and the field survey. GT played a major role in carrying out this study in the field and assisted in the writing part.
Acknowledgements: We sincerely thank our professor and guide Dr. Advait Edgaonkar, Faculty at Indian Institute of Forest Management, Bhopal for helping throughout during our study, for providing his valuable inputs at all the stages - field survey, data analysis and paper writing.

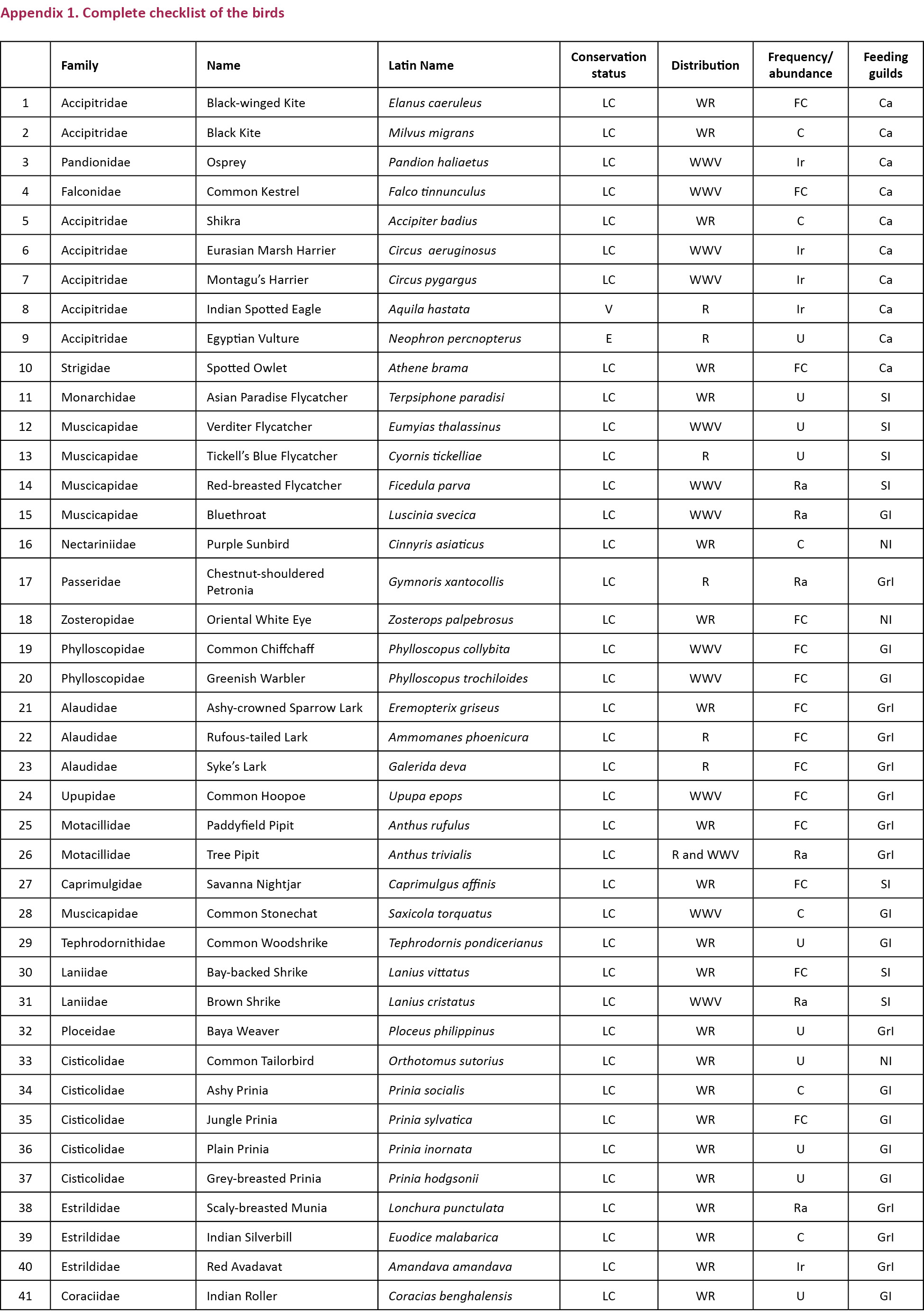
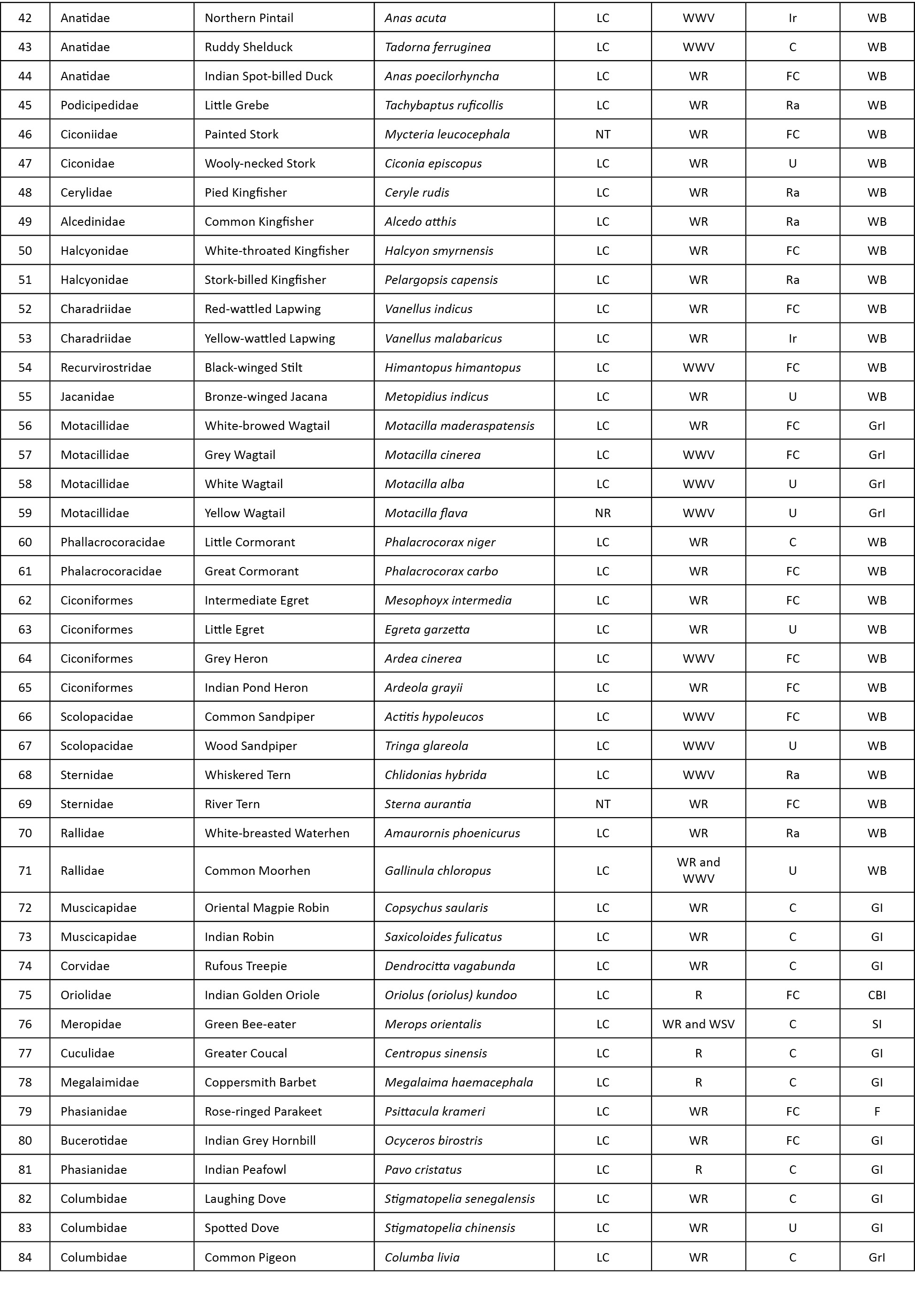
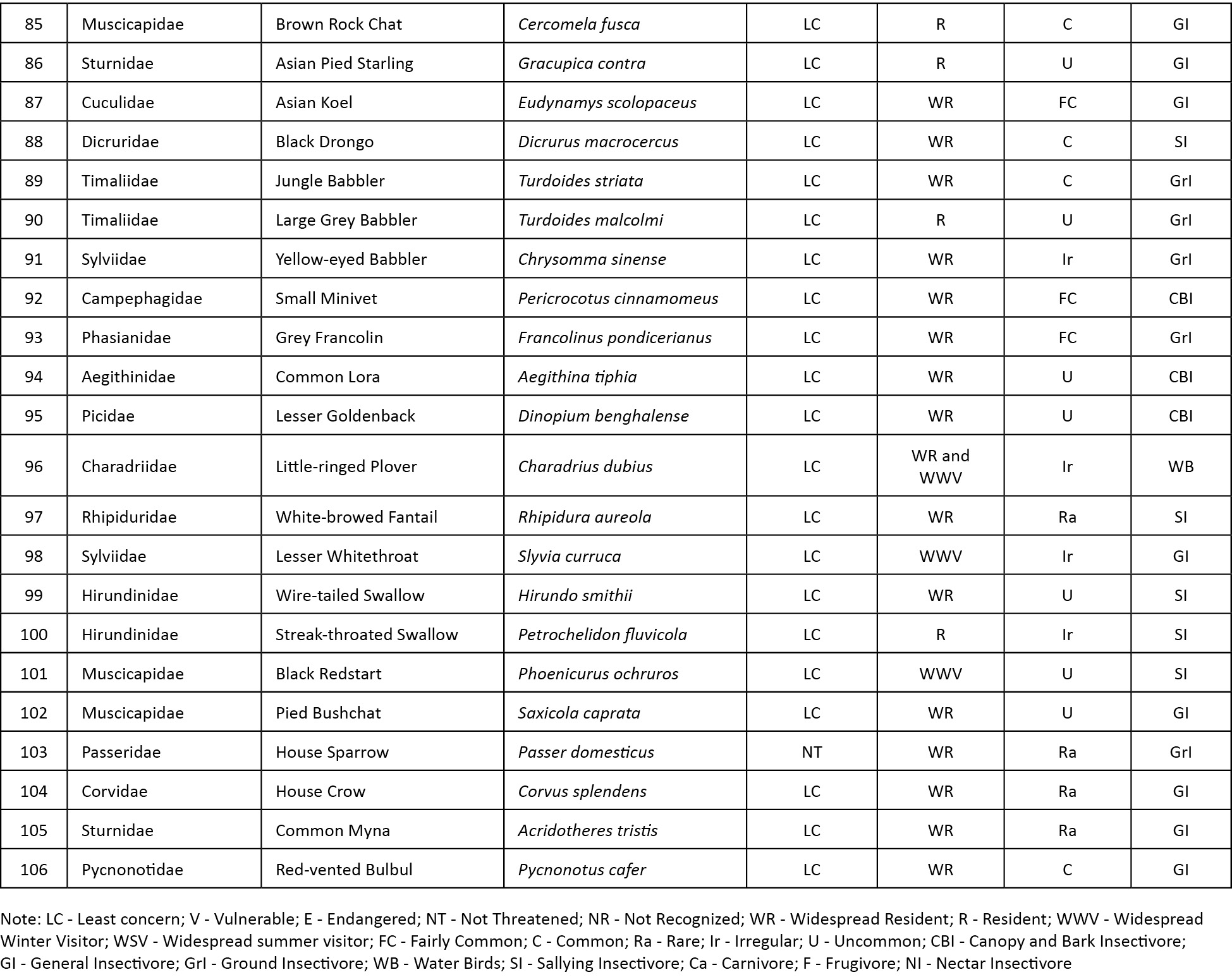
Abstract: A study to find out the bird diversity at the Indian Institute of Forest Management (IIFM), Bhopal, was carried out over a period of nine months from July 2012 to March 2013. IIFM is located on a hill facing Bhadbhada barrage in Bhopal. Physiographically the area is classified as Vindhayan Hills. A total of 106 bird species belonging to 52 families were recorded during the study covering an area of about 93 hectares. The study area was divided into three major habitat types: open scrub, dry deciduous, and urbanized. Bird species were classified into eight feeding guilds: carnivore, ground insectivore, sallying insectivore, canopy and bark insectivore, nectar insectivore, general insectivore, frugivore and water birds. Of the total 106 species observed, 27 species were recorded as winter visitors. Density analysis was done using DISTANCE software and density was found out to be 32.7 birds per hectare. Rank abundance curve was used for assessing species composition in different habitats and during different seasons. In terms of both richness and evenness, open scrub scored the highest rank (72 species, and most even distribution of species). Higher species richness with lower species evenness was recorded during winter season for all the habitats.
Keywords: Bhopal, density, avian diversity, evenness, feeding guilds, habitat, richness.
Abbreviations: AIC- Akaike information criterion; C - Carnivore; C&B - Canopy and bark insectivore; F - Frugivore; GI - General insectivore; GrI - Ground insectivore; NI - Nectar insectivore; SE - Standard error; SI - Sallying insectivore; WB - water birds.
Introduction
Birds are some of the most prominent species of the Earth’s biodiversity and being sensitive to environmental changes they act as key indicators for assessing the status of ecosystem health (Taper et al. 1995; Olechnowski 2009). Assessing the bird diversity of a habitat over time and space is one of the key issues for avian community ecologists. Richness, abundance and community composition are often used by ecologists to understand the diversity of species in their natural occurrence (Magurran 2004).
The bird community structure is affected by changes in vegetation structure either due to natural or any human induced disturbances (Maurer 1981; Wiens 1989; Rahayuninagsih et al. 2007). Talking about disturbances when two such disturbances occur simultaneously or in quick succession they might lead to a compound disturbance that by impacting ecological resilience and recovery (Buna & Wessman 2011; Harvey et al. 2014) may result in ecological surprises (Paine et al. 1998). Wild fire and simultaneous outbreak in insects like bark beetle can be categorized as one such disturbance. Contrary to general belief of this disturbance having a negative impact on the abundance of bird species, many studies have found that bird species were more abundant post fire when compared with burn free areas (Hutt 1999; Kotliar et al. 2002). This also leads to a change in composition of bird species by addition of certain sallying, canopy and bark insectivorous species.
The change in vegetation composition could impact the quality and quantity of habitat for birds in terms of food, water and cover which can further affect the diversity, abundance and distribution of birds (Western & Grimsdell 1979).
In order to prioritize the future conservation of species, understanding the effect of habitat on bird community structure is important (Zakaria et al. 2011). In the long run, the relative value of different habitats and conservation importance of sites can be assessed by investigating the diversity of birds present at those sites (Bensizerara et al. 2013).
Many researchers have already documented the response that avian diversity shows to different vegetation composition structure (MacArthur & MacArthur 1961), and have also demonstrated that avian diversity increases with an enhanced level of vegetation (Wiens 1969).
This study aims to investigate the bird community structure, bird diversity and density at the IIFM campus. An effort has also been made to prepare a checklist of its bird species. In this paper the bird community structure and composition in different habitats of the campus has been documented. The study also demonstrates the change in density and composition of bird species after an outbreak of fire. There are areas in the campus where human disturbances like logging, grazing are being practiced. The study will assess the differences in avian community among these areas and how are they being impacted by them.
Materials and Methods
Study area
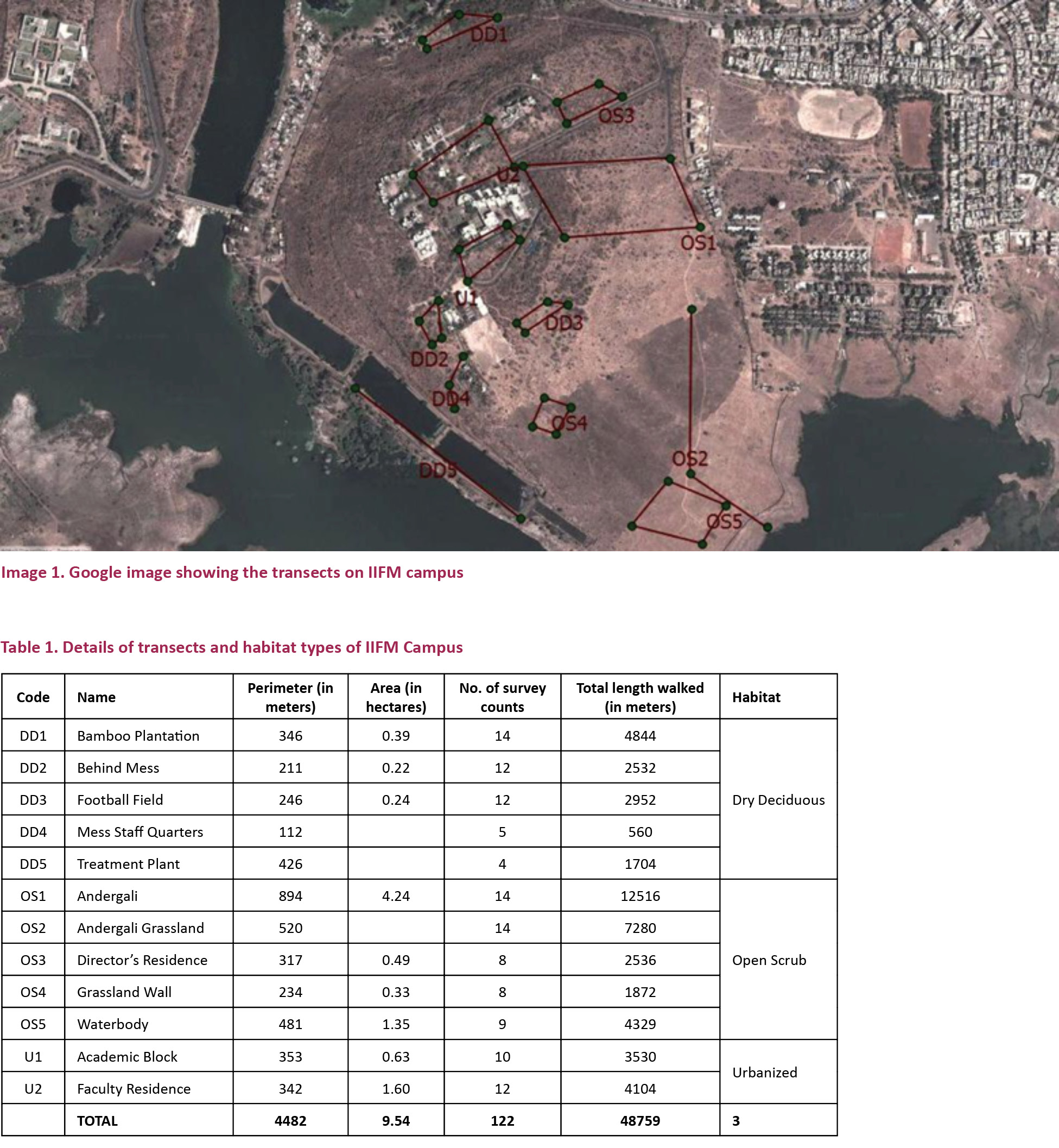
The study was done in the Indian Institute of Forest Management, Bhopal (23.2083710N & 77.3844170E), from July 2012 to March 2013. The location of the campus, built on a hill surrounded by water on three sides, along with a wide range of climatic conditions that it passes through brings in diverse structure of habitats. The major types of vegetation include grasslands, open scrub forest, dry deciduous forest and bamboo groves. The study was conducted in 12 transects covering an area of 93 hectare campus (Image 1).
For our study, transects were divided into three habitats according to general landscape attributes and vegetation present there. The chief habitat types were:
(i) Open scrub comprising mainly grasslands and scarce vegetation of Leucaena leucocephala.
(ii) Dry deciduous comprising grass species, Hardwickia binata, scrubs, Azadirachta indica.
(iii) Urbanized human inhabited areas like the faculty block, academic block and so on.
The details about transects and habitats into which the campus was divided is given in Table 1. These habitats are also structured by different levels of human disturbance varying from activities like logging, cattle grazing, human settlements and presence of domestic dogs.
Bird survey
The bird population was recorded using the belt transect method (Cunningham et al. 2006). During a transect walk, the observer recorded data on the sightings of bird species, number of individuals sighted and perpendicular distance from the line at which the species was sighted. Only those observations lying within 20m of either side of the transect line were recorded. The survey was conducted either during the morning time zone (between 07:00–09:00 hr) or during the evening time zone (between 16:00–18:00 hr) when there is maximum bird activity (Cunningham et al. 2006; Simons et al. 2006).
Feeding guilds
The study of avian feeding guilds is important for understanding the complexity of ecosystem structure and for providing updated information on each type of habitat in the ecosystem (Azman et al. 2011). A feeding guild may be defined as a group of species that have similar feeding or foraging habits (Hutto 1985).
Eight feeding guilds were identified in the study area: carnivore (C), frugivore (F), canopy and bark insectivore (C&B), general insectivore (GI), ground insectivore (GrI), nectar insectivore (NI), sallying insectivore (SI) and water birds (WB).
Density estimation
The Distance sampling method was used to estimate bird density using detection probability as a function of distance (Simons et al. 2006; Somershoe et al. 2006; Broekema & Overdyck 2012). The detection function was fitted for uniform models with cosine and simple polynomial function as well as half-normal models with cosine and hermite polynomial expansion. AIC value was further used to select the best fitting model. DISTANCE 6.0 was used to calculate density estimates. (Norvel et al. 2003).
For this study, the data was segmented on the basis of season - monsoon (from August to September), autumn (from October to November), winter (from December to 2nd week of February) and spring (from 3rd week of February to March) and on the basis of feeding guilds within different habitat types.
Bird species diversity and abundance
To assess the distribution and differences in the abundance of birds between different habitats, the rank abundance curve (Magurran 1988) was plotted to assess the structure of bird communities in different habitats using the percent of total sample for each species as its index of abundance (Simons et al. 2006). Rank abundance curves were generated for avian assemblages at each site. The diversity of site increases as the slope of curve approaches zero. Steepness of the slope defines evenness at a site; if it is less steep it means a more even distribution of species.
Results
We recorded a total of 106 species of birds, in which, 10 carnivore, 21 ground insectivore, 27 general insectivore, one frugivore, four canopy and bark insectivore, three nectar insectivore, 13 sallying insectivore and 27 water birds were recorded.
Density estimate
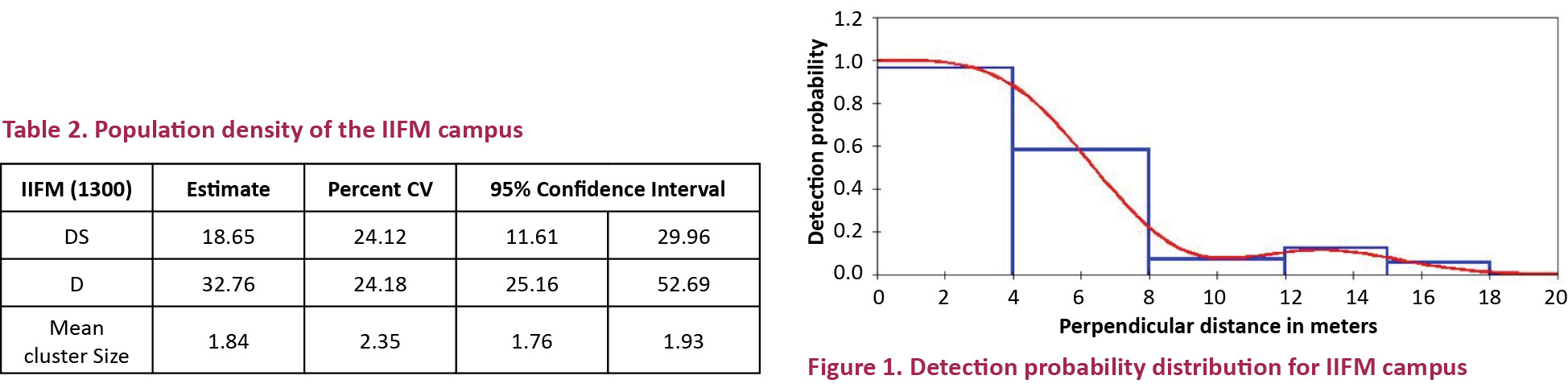
A half normal key estimator was selected by DISTANCE as detection model. The result was found for the whole campus in terms of density of clusters, individual density and mean cluster size (Table 2).
The population density for cluster was found to be 18.64 (SE=4.49) birds per hectare while individual density was found to be 32.75 (SE=7.92) per hectare. The mean cluster size was 1.84 birds per hectare. Fig. 1 shows the detection probability distribution for the whole campus.
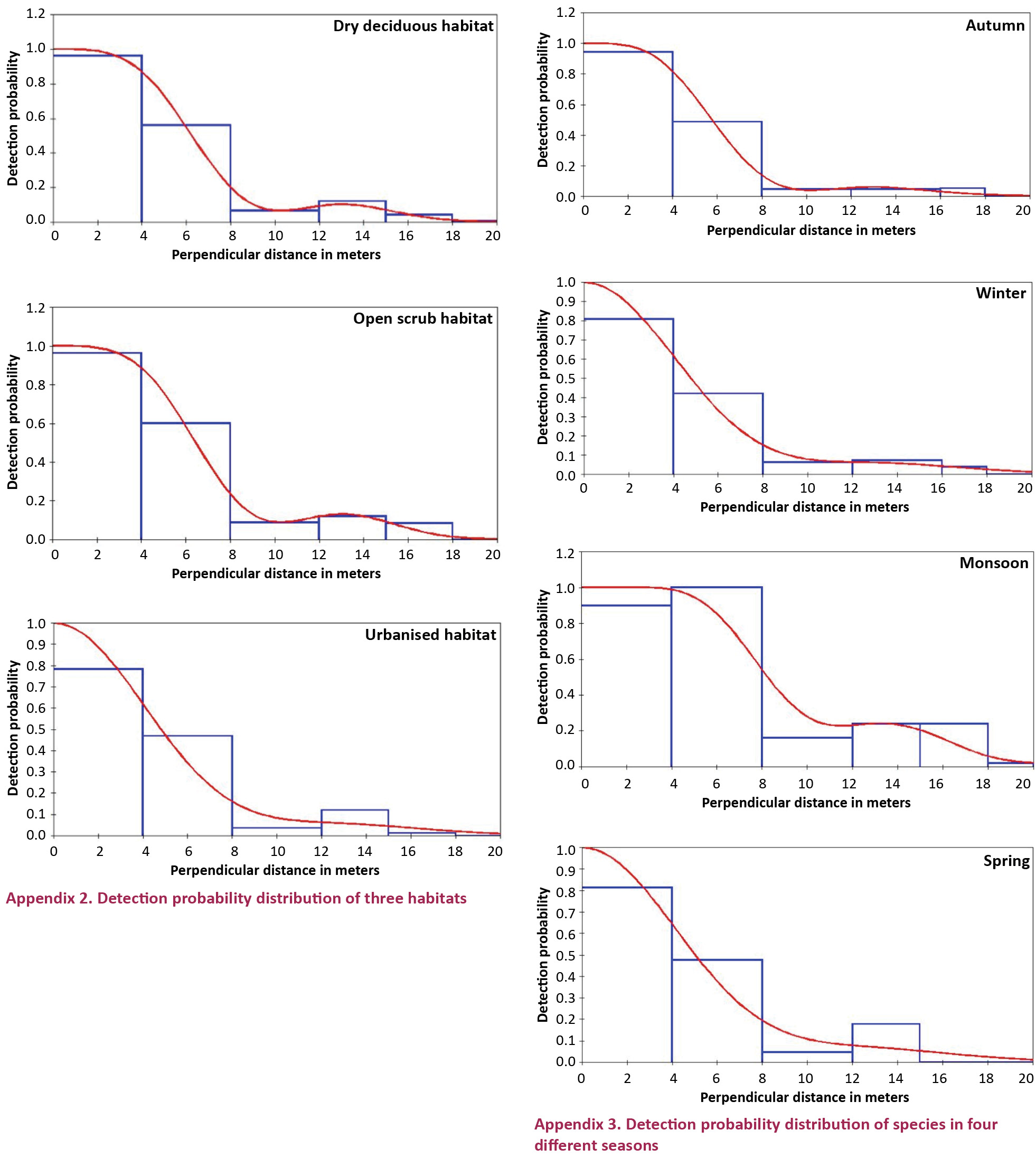
The population was further estimated on the basis of habitat and seasons (Tables 3, 4). In the dry deciduous habitat, which combined DD1 to DD5 transects, the population density for the cluster was found to be 36.22 (SE 11.67) birds per hectare while individual density was found to be 60.52 (SE 19.55) per hectare. The mean cluster size was 1.64 birds per hectare. In open scrub, which combined OS1 to OS5 transects, the population density for the cluster was found to be 14.52 (SE 3.88) birds per hectare while individual density was found to be 25.79 (SE 6.91) per hectare. The mean cluster size was 1.93 birds per hectare. In the urbanized habitat which combined U1 and U2 transects, the population density for the cluster was found to be 13.82 (SE 2.28) birds per hectare while individual density was found to be 26.81 (SE 4.72) per hectare. The mean cluster size was 2.15 birds per hectare. Detection probability distribution for all three habitats can be found in Appendix 2 which shows that distribution was quite similar in the dry deciduous and the open scrub habitat compared to that of the urbanized habitat.
In the autumn season the density of birds was found to be 12.65 birds per hectare while the mean cluster size was estimated to be 1.93. For the winter season the density of birds was found to be 14.93 birds per hectare with the mean cluster size as 1.82. For the monsoon season the density was estimated to be 2.54 birds per hectare with the mean cluster size of 1.63. In the spring the density was found to be 3.42 birds per hectare and the mean cluster size was found to be 1.92. Appendix 3 shows the detection probability distribution in all the four seasons in campus which indicates that the autumn and the winter have quite similar probability distribution compared to the monsoon and the spring’s distribution.
Bird species diversity
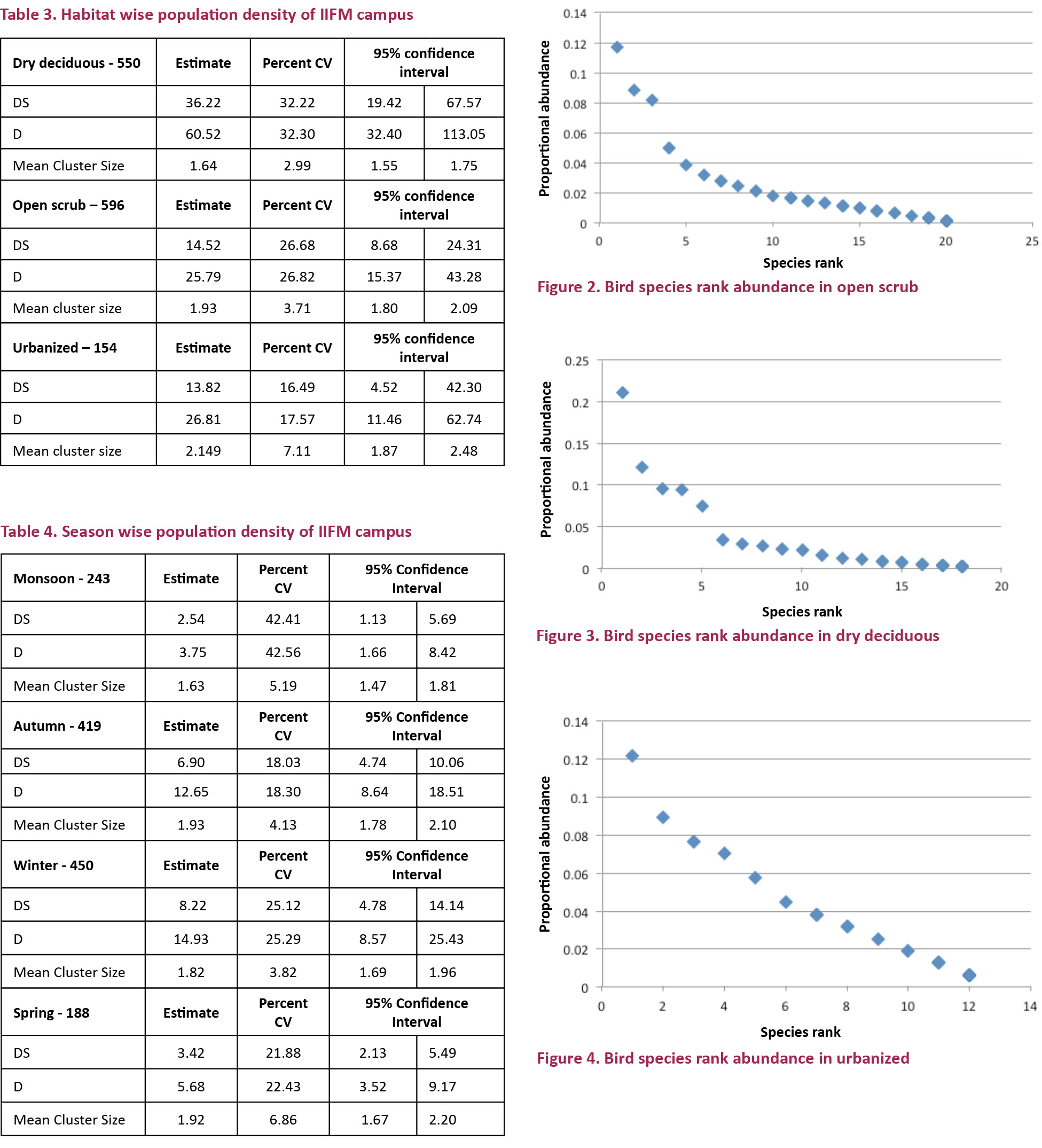
The rank abundance curve indicates that the species diversity and composition differed in the entire habitat. The open scrub, with the highest number of individuals (596) and the highest number of species (72) ranks as the most diverse habitat as the distribution is also very even (Fig. 2). The number of individuals in the dry deciduous was 550 with 51 species while in the urbanized habitat, the number of individuals was 156 with 35 species (Figs. 3, 4).
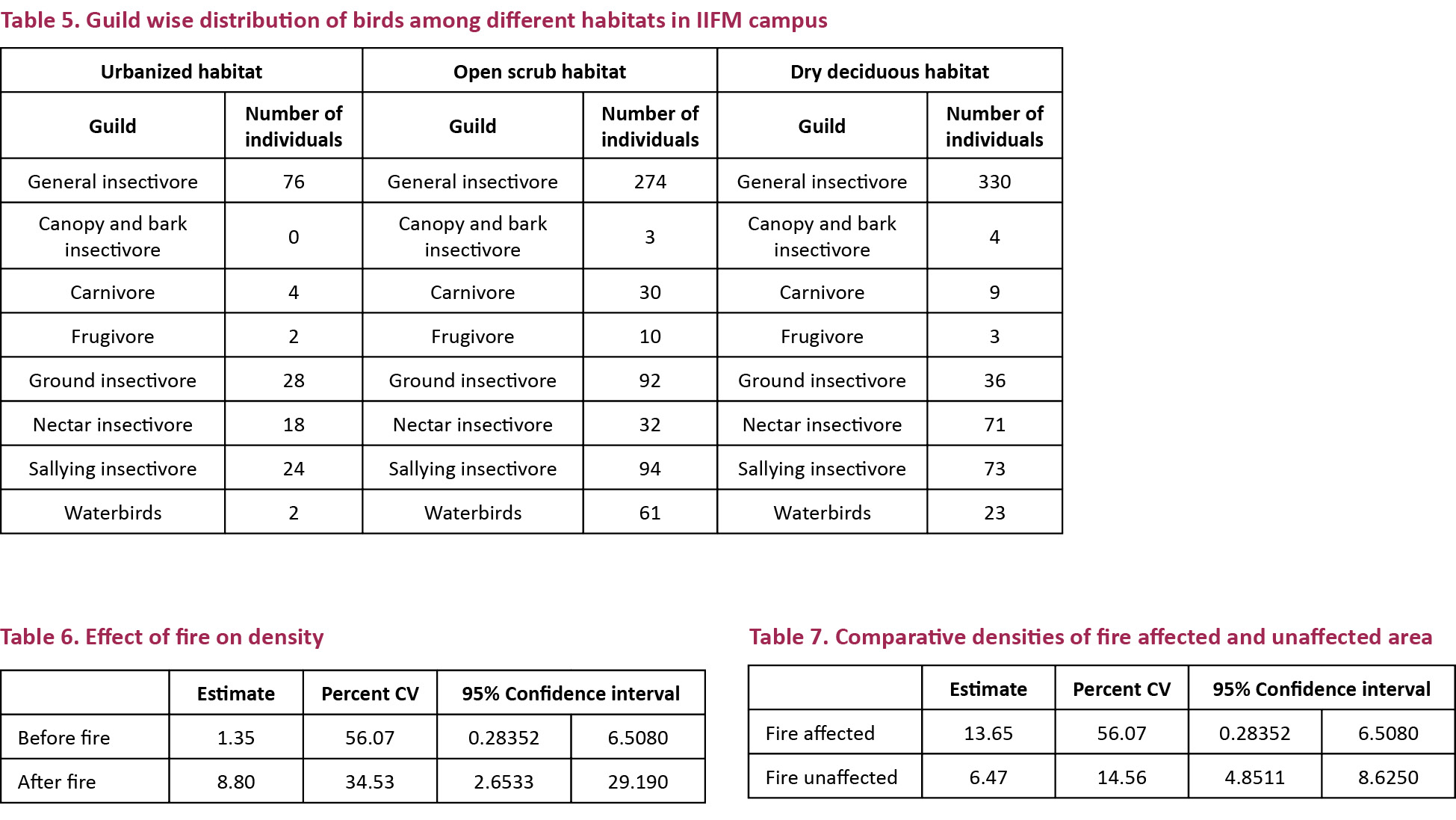
Thirty-eight species were common to both the open scrub and the dry deciduous. Open scrub was populated with a higher number of species belonging to the carnivore and the ground insectivore guild while the dry deciduous was populated with more species in the nectar insectivore guild, with the rest remaining almost the same. In the urbanized habitat, there were no species belonging to the canopy and bark insectivore guild and relatively a very low number of individuals in other categories too (Table 5).
Density estimate during an outbreak of fire in the open scrub habitat
Before the fire took place, the density of birds was found to be 1.35 birds per hectare while after this event the density increased to 8.80 birds per hectare (Table 6).
Table 7 shows the density of birds in the fire affected area and in the area unaffected by fire one month after the outbreak of fire. Fire affected area had density of 13.65 birds per hectare while fire unaffected area had density of 6.47 birds per hectare (Table 7).
Discussion
The present study produced a reliable estimate of birds through direct observations on line transects that were repeatedly walked for over a considerable period of time (Anderson 1983; Kumara 2012; Laure 2007).
The campus has a rich variety of strata and guilds owing to its topography and different habitats like water bodies, open scrub area, dry deciduous area and areas of human settlement, which enhanced the diversity of birds.
In terms of bird community structure, it was largely similar in the open scrub and the dry deciduous habitats as compared to that of the urbanized habitat. The open scrub and the dry deciduous shared 38 common species. There were more numbers of ground insectivore in the open scrub than in the dry deciduous. This high number of species was attributed to the forest fire (Raphael et al. 1987; Adeney et al. 2006; Martin et. al 2006) that took place in October, 2012 in the open scrub. It also affected the diversity of the grassland in terms of a reduction in sightings of nightjars and the addition of a whole new feeding guild of ground insectivores. This was the direct result of an increase in the number of insects after the fire (Russell et al. 2009) which led to the arrival of birds like larks—Ashy Crowned Sparrow Lark Eremopterix griseus, Rufous-tailed Lark Ammomanes phoenicura, Oriental Sylark Alauda gulgula, Skykes’s Lark Galerida deva, Indian Bushlark Mirafra erythroptera in groups of 10–20, Common Hoopoe Upupa epops, and Pipits—Paddyfield Pipit Anthus rufulus, Tree Pipit Anthus trivialis. The analysis of the data of one month before and one month after the fire showed an increase in density by 6.51 times after the fire took place.
To account for changes in the density due to seasonal variations, the after fire density of two adjacent transects, i.e., one affected by the fire and one not affected by the fire was carried out. Both of these transects were in the grassland area. The data taken into consideration was for the duration of one month after the fire.
The bird density in the fire affected area was 2.11 times greater than the unaffected area for the same duration for the same habitat.
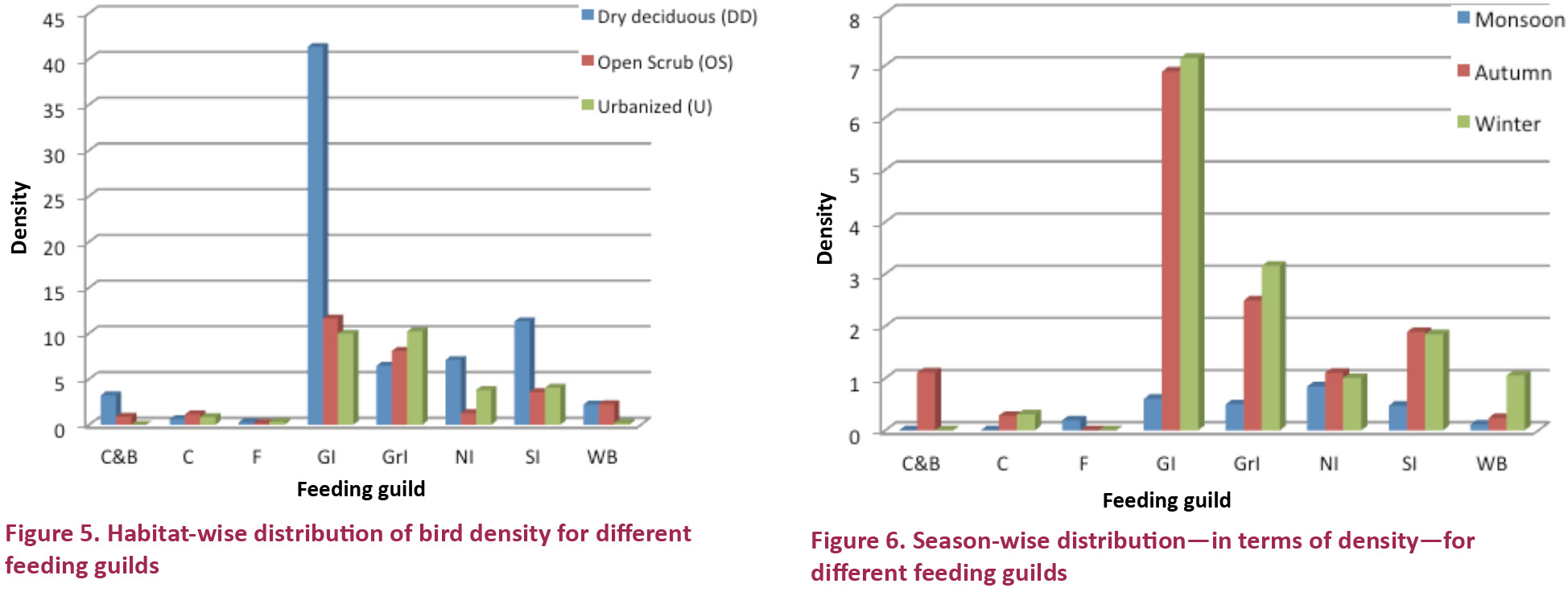
Habitat to a large extent determined the species composition (Fig. 5). Out of the three studied habitats, the dry deciduous habitat was found to have a maximum density of canopy and bark insectivores. This can be attributed to the presence of suitable tree species in the habitat apart from its close proximity to an agricultural area (Abdar 2014).
The presence of canopy and bark insectivores can also be favored by the presence of appropriate vegetation cover with trees having appropriate dimensions for different activities of these species (Rajpar & Zakaria 2011).
The urbanized habitat showed the highest density of ground insectivores due to the abundant presence of Jungle Babblers Turdoides striata in the academic block and faculty residence transects, which can be attributed to the presence of tree species providing appropriate fruit and seed to the species. Black Drongo Dicrurus macrocercus, Ashy Prinia Prinia socialis were seen in all the transects and the habitat types. Red-vented Bulbul Pycnonotus cafer, Jungle Babbler Turdoides striata were seen all over the major habitat types except the grassland area. Their presence all over the campus can be explained on the basis of their generalist nature and good adaptation capability to any environment and even to human settlements (Sharat et al. 2011).
The water birds were mostly recorded from the open scrub habitat due to the presence of the Bhadbhada barrage surrounding the grassland area of IIFM.
The differences in bird community structure among the habitats can also be explained by the different levels of human disturbances experienced by these habitats. Among the three habitats, the urbanized area experienced the highest level of disturbance mostly due to the location of this habitat. Between the open scrub and the dry deciduous, the latter was more disturbed due to the high level of logging and cattle grazing (Gregory et al. 2010). Logging can change forest structure in terms of an increase in gap areas and a decrease in cover (Johns 1985). Birds are generally the first group of vertebrates to respond to any logging (Johns 1988). Many studies have reported changes in species diversity with higher species richness in less disturbed forests as compared to disturbed forests (Johns 1985, 1991; Thiollay 1992; Riffell et al. 1996; Marsden 1997). This also supports the result that we obtained in our study in terms of different bird community structure.
Barring a few exceptions, the maximum feeding guild density was observed in the winter season (Fig. 6). The canopy and bark insectivores were found to be at a maximum in the autumn season.
The frequency at which Rose-ringed Parakeet Psittacula krameri was seen was the greatest in the Monsoon season which gradually reduced towards the winter season. During the winter season species like Common Kestrel Falco tinnunculus and Harrier spp. Eurasian Marsh Harrier Circus aeruginosus, Montagu’s Harrier Circus pygargus, Pallid Harrier Circus macrourus visited the campus, particularly in the open scrub habitat. These birds, apart from being winter visitors (Riegert 2005), were also favored by the outbreak of fire which took place in the open scrub habitat in October owing to the creation of open areas and suitable perching points to capture aerial prey and small mammals in open areas (Wiles et al. 2000; Narwade et al. 2011; Himanshu et al. 2012).
Conclusion
In the present study we have showed that seasonality along with change in the habitat structure may influence bird assemblage organization over time. The abundant populations of the Jungle Babbler, the Black Drongo and the Red-vented Bulbul shows an increasing urbanization in the vicinity and even inside the campus. An increase in human settlements will cause more danger to avian species. A more eco-friendly urbanization is essential to curb any more negative human interferences in the areas. Further research on appropriate conservation mechanisms and management techniques with the ultimate conservation goal of changing urban environments into species rich ecosystems are required.
References
Abdar, M.R. (2014). Seasonal Diversity of Birds and Ecosystem Services in Agricultural Area of Western Ghats, Maharashtra State, India.Journal of Environmental Science, Toxicology And Food Technology 8(1): 100–105.
Adeney, J.M., J.R. Ginsberg, G.J. Russell & M.F. Kinnaird (2006). Effects of an ENSO-related fire on birds of a lowland tropical forest in Sumatra. Animal Conservation 9(3): 292–301.
Azman, M.N., N.S. Latip, M.S. Sah & N.J. Shafie (2011). Avian Diversity and Feeding Guilds in a Secondary Forest, an Oil Palm Plantation and a Paddy Field in Riparian Areas of the Kerian River Basin, Perak, Malaysia. School of Biological Sciences. Universiti Sains Malaysia, 22(2) 45–64.
Bensizerara, D., H. Chenchouni, A.S. Bachir & M. Houhamdi (2013).Ecological status interactions for assessing bird diversity in relation to a heterogeneous landscape structure. Avian Biology Research 6(1): 67–77.
Broekema, I. & O. Overdyck (2012). Distance sampling to estimate densities of four native forest bird species during multi species surveys. New Zealand Journal of ecology 36(3): 353–364.
Buma, B. & C.A. Wessman (2011). Disturbance interactions can impact resilience mechanisms of forests. Ecosphere 2:1–13.
Cunningham, M.A., D.H. Johnson & D.N. Svingen (2006). Estimates of Breeding Bird Populations in the Sheyenne National Grassland, North Dakota. The Prairie Naturalist 38(1): 50–67.
David, R. & K.P. Anderson (1983). Density estimation of small mammal populations using a trapping web and distance sampling methods. The Ecological Society of America 64(4): 674–680.
Gregory, N.C., R.L. Sensenig & D.S. Wilcove (2001). Effects of controlled fire and livestock grazing on bird communities in East African savannas. Conservation Biology 24: 1606–1616.
Harvey, B.J., D.C. Donato, W.H. Romme & M.G. Turner (2014). Fire severity and tree regeneration following bark beetle outbreaks: the role of outbreak stage and burning conditions. Ecological Applications 24: 1608–1625; http://dx.doi.org/10.1890/13-1851.1
Himanshu, S.P., P.M. Pratyush & K.S. Hemanta (2012). Birds of Hadagarh Wildlife Sanctuary, Odisha, Eastern India. World Journal of Zoology 7(3): 221–225.
Hutto, R.L. (1985). Habitat Selection in Birds. Academic Press. Inc., Montana, 455.
Hutto, R.L. (1995). Composition of bird communities following stand-replacement fires in northern rocky mountain (USA) conifer forests. Conservation Biology 9: 1041–1058.
Johns, A.D. (1985). Selective logging and wildlife conservation in tropical rain forest: Problems and recommendations. Biological Conservation 31: 355–375
Johns, A.D. (1988).Effects of selective timber extraction on rainforest structure and composition and some consequences for frugivores and folivores. Biotropica 20(1): 31–37.
Kotliar, N.B., S.J. Hejl, R.L. Hutto, V.A. Saab, C.P. Melcher & M.E. Mcfadzen (2002). Effects of fire and post fire salvage logging on avian communities in conifer dominated forests of the western United States. Studies in Avian Biology 25: 49–64.
Kumara, S.R. (2012). Estimating Asian Elephant Elephas maximus, density through distance sampling in the tropical forests of Biligiri Rangaswamy Temple Tiger Reserve, India. Tropical Conservation Science 5(2): 163–172.
MacArthur, R.H. & J.W. MacArthur (1961). On bird species diversity.Ecology 42: 594–598.
Magurran, A.E. (1988). Ecological Diversity and its Measurement.Princeton University Press, Princeton, NJ, 192pp.
Marsden, S.J. (1997). Changes in bird abundance following selective logging on Seram, Indonesia. Conservation Biology 12(3): 605–611.
Martin, K., A. Norris & M. Drever (2006).Effects of bark beetle outbreaks on avian biodiversity in the British Columbia interior: Implications for critical habitat management.BC Journal of Ecosystems and Management 7(3): 10–24.
Maurer, B.A., L.B. McArthur & R.C. Whitmore (1981). Effect of logging on guild structure of a forest bird community in West Virginia.Ecology 35: 11–13.
Michelle, A.L., C.K. Nielsen & M.D. Grund (2007). Using Distance Sampling to Estimate Densities of White-Tailed Deer in South-Central Minnesota. The Prairie Naturalist 39(2): 57–68.
Narwade, S. & M.M. Fartade (2011). Birds of Osmanabad District of Maharashtra, India. Journal of Threatened Taxa 3(2): 1567–1576; http://dx.doi.org/10.11609/JoTT.o2462.1567-76
Norvel, R.E., F.P. Howe & R. Jimmie (2003). A seven-year comparison of relative-abundance and distance-sampling methods. The Auk 120(4): 1013–1028.
Olechnowski, B.F. (2009). An examination of songbird avian diversity, abundance trends, and community composition in two endangered temperate ecosystems: riparian willow habitat of the Greater Yellowstone Ecosystem and a restored tallgrass prairie ecosystem, Neal Smith National Wildlife RefugeIowa State University. Iowa State University.
Paine, R., M. Tegner & E. Johnson (1998). Compounded perturbations yield ecological surprises. Ecosystems 1: 535–545.
Rahayuninagsih, M., A. Mardiastuti, L. Prasetyo & Y. Mulyani (2007). Bird community in Burungisland, Karimunjawa National Park, Central Java. Biodiversity 8: 183–187.
Rajpar, M.N. & M. Zakaria (2011). Bird species abundance and their correlationship with microclimate and habitat variables at Natural Wetland Reserve, Peninsular Malaysia. International Journal of Zoology 2011: 17pages; http://dx.doi.org/10.1155/2011/758573
Raphael, M.G., M.L. Morrison & M.P. Yoder-Williams (1987). Breeding bird populations during twenty-five years of postfire succession in the Sierra Nevada. The Condor 89: 614–626.
Riegert, J. (2005). Ecology of urban Common Kestrels (Falco tinnunculus).PhD Thesis. University of South Bohemia, ČeskéBudějovice.
Riffell, S.K., K. Gutzwiller, A. Jhons & H. Stanley (1996). Does repeated human intrusion cause cumulative declines in avian richness and abundance? Ecological Applications 6(2): 492–505.
Russell, R.E., A. Royle, V. Saab, J. Lehmkuhl, W. Block & J. Sauer (2009). Modeling the effects of environmental disturbance on wildlife communities: avian responses to prescribed fire. Ecological Applications 19: 1253–1263.
Sharat, K.P., V.P. Aditya & D. Uppeander (2011). Habitat enrichment and its impact on avian diversity: a study at GBPIHED, Kosi-Katarmal, Uttarakhand, India. Current Science 100: 11–14.
Simons, T.R., S.A. Shriner & G.L. Farnsworth (2006). Comparison of breeding bird and vegetation communities in primary and secondary forests of Great Smoky Mountains National Park.Biological Conservation 129: 302–311.
Somershoe, S.G., D.J. Twedt & R. Bruce (2006). Combining breeding bird survey and distance sampling to estimate density of migrant and breeding birds. The Condor 108: 691–699.
Taper, M.L., K. Bohning-Gaese & J.H. Brown (1995). Individualistic responses of bird species to environmental change. Oecologia 101: 478–486.
Thiollay, J.M. (1995). The role of traditional agroforests in the conservation of rain forest birds diversity in Sumatra. Conservation Biology 9(2): 335–353.
Western, D. & J.J.R. Grimsdell (1979). Measuring the distribution of animals in relation to the environment. Handbook No. 2, African Wildlife Leadership Foundation, Nairobi.
Wiens, J.A. (1969). An approach to the study of ecological relationships among grassland birds. Ornithological Mongraphs 8: 1–93.
Wiens, J.A. (1989). The Ecology of Bird Communities. Process and Variation - Vol. 2. Cambridge University Press, 316pp
Wiles, G.J., D.J. Worthington, R.E. Beck, H.D. Pratt, C.F. Aguon & R.L. Pyle (2000). Noteworthy bird records for Micronesia, with a summary of raptor sightings in the Mariana Islands, 1988–1999. Micronesica 32(2): 257–284.