Pollination ecology of Chengam Scyphiphora hydrophyllacea C.F. Gaertn. (Magnoliopsida: Rubiales: Rubiaceae),
a non-viviparous evergreen tree species
A.J. Solomon Raju 1 & B. Rajesh 2
1,2 Department of Environmental Sciences, Andhra University, Visakhapatnam, Andhra Pradesh 530003, India
1 ajsraju@yahoo.com (Corresponding author), 2 raj27prabha@gmail.com
doi: http://dx.doi.org/10.11609/JoTT.o3998.6668-76
Editor: V. Sampath Kumar, Botanical Survey of India, Howrah, India. Date of publication: 26 December 2014 (online & print)
Manuscript details: Ms # o3998 | Received 16 April 2014 | Final received 30 November 2014 | Finally accepted 03 December 2014
Citation: Raju, A.J.S. & B. Rajesh (2014). Pollination ecology of Chengam Scyphiphora hydrophyllacea C.F. Gaertn. (Magnoliopsida: Rubiales: Rubiaceae), a non-viviparous evergreen tree species. Journal of Threatened Taxa 6(14): 6668–6676; http://dx.doi.org/10.11609/JoTT.o3998.6668-76
Copyright: © Raju & Rajesh 2014. Creative Commons Attribution 4.0 International License. JoTT allows unrestricted use of this article in any medium, reproduction and distribution by providing adequate credit to the authors and the source of publication.
Funding: Ministry of Environment, Forests and Climate Change, Government of India, new Delhi (File No. 22-34/2009-CS).
Competing Interest: The authors declare no competing interests.
Author Contribution: AJSR conceived the project, did part of field work and prepared the paper. BR did field work and tabulated the observational and experimental work of the paper.
Author Details: Prof. A.J. Solomon Raju is the Chairman, Board of Studies, Department of Environmental Sciences, Andhra University, Visakhapatnam. He is presently working on endemic and endangered plant species in southern Eastern Ghats forests with financial support from MoEF and CSIR. B. RAJESH is working as Junior Research Fellow in the MoEF Research Project under Prof. A.J. Solomon Raju. He has registered for PhD under Prof. Solomon Raju.
Acknowledgements: This study is a part of the research work carried out under a Major Research Project entitled “Reproductive biology of some mangrove plant species at Coringa Mangrove Forest” (MoEF No. 22-34/2009-CS) funded by the Ministry of Environment & Forests, New Delhi sanctioned to AJSR. The second author is JRF working in this project. We thank Dr. K. Venkanna, Technical Officer, Central Research Institute for Dry land Agriculture, Hyderabad, for doing soil physico-chemical analysis.
Abstract: Scyphiphora hydrophyllacea C.F. Gaertn. or Chengam is a non-viviparous evergreen tree species. The flowers are bisexual, self-compatible, self-pollinating, temporally dioecious and exhibit a mixed breeding system. The plant is both melittophilous and anemophilous at the study area. Natural fruit set is 100% but seeds are non-viable which might be due to a genetic disorder.
Keywords: Anemophily, melittophily, mixed breeding system, pollination, regeneration absence.
INTRODUCTION
Scyphiphora C.F. Gaertn. is a monotypic genus belonging to the family Rubiaceae. The species, Scyphiphora hydrophyllacea C.F. Gaertn., known as Chengam, is an uncommon constituent of mangroves and distributed from southern India and Sri Lanka throughout Southeast Asia to northern Australia and western Polynesia (Solomon Islands). It occurs in muddy, sandy and rocky substrates on the landward margin of mangroves or on the banks of tidal waterways. It has been reported to be intolerant of lengthy periods of freshwater inundation and usually occupies sites that are frequently inundated by the tide (Heyne 1950; Tomlinson 1986; Wim et al. 2006; Tao and Charlotte 2011). The species has been reported to be declining in many regions primarily due to extraction and coastal development. In this context, Ellison et al. (2010) stated that it is not enough to include this species under any of the threatened category thresholds globally and hence is listed as Least Concern. These authors also reported that this species appears in small numbers in most areas of its range. It is considered rare in India and Sri Lanka. Hettiarachchi et al. (2002) reported that it is a highly threatened species in Sri Lanka. Rao et al. (1998) assessed the status of this species as Endangered due to its threatened restricted distribution in India in the CAMP workshop on national assessment of mangrove flora and fauna. Ramasubramanian et al. (2003) noted that it is a rare species in Coringa mangrove forest, Andhra Pradesh, India. S. hydrophyllacea has been reported to be self- or insect-pollinated by Wim et al. (2006), insect-pollinated by Wheeler et al. (1992) and bee-pollinated by Tomlinson (1986) and Selvam and Karunagaran (2004). Further, Wheeler et al. (1992) briefly mentioned that the pollination mechanism in this species is conspicuously specialized with passive pollen presentation involving stylar modification. Puff & Rohrhofer (1993) reported that the flowers possess “ixoroid” pollination mechanism, in which the flowers are protandrous and deposit the pollen on the outside of the stigmas and style for dispersal. Since this sporadic information does not provide any clarity to understand the reproductive bottlenecks with reference to its breeding system, pollination system and regeneration ecology, the present study attempts to provide details of pollination ecology of this species to understand its sexual reproduction and regeneration difficulties in its natural areas.
MATERIALS AND METHODS
Study site
The Coringa Mangrove forest is located in the Godavari Estuary in the East Godavari District of Andhra Pradesh, between 16039”–17000”N and 82014”–82023”E. The total area of the mangrove cover is 316km2 of which 235.7km2 is under Coringa Wildlife Sanctuary. This sanctuary comprises reserve forests, viz., Coringa R.F., Coringa Extension R.F. and Bhairavapalem R.F. The mangrove cover in these three reserve forests is not directly connected with the Bay of Bengal. The non-sanctuary mangrove area has six reserve forests, namely, Rathikalava, Masanitippa, Matlatippa, Balusutippa, Kothapalem and Kandikuppa. This non-sanctuary area is connected to the sea and interestingly, Scyphiphora hydrophyllacea occurs only in Kothapalem Reserve Forest. Soil analysis was done for certain chemical characters such as pH, EC, OC, N, P, K and physical character soil texture to evaluate the nutrient status of the soil. The analysis was carried out at Central Research Institute for Dry land Agriculture, Hyderabad.
Phenology and floral biology
The work was carried out from 2012 to 2014. Intensive field studies were conducted at weekly intervals during flowering and fruiting seasons. The morphological characters of the flower were described based on 25 flowers collected randomly from five trees. Quantification of the number of flowers produced per inflorescence and the duration of inflorescence life were determined by tagging 10 inflorescences, which have not initiated flowering, selected at random and following them daily until they ceased flowering permanently. Anthesis was initially recorded by observing marked mature buds. Later, the observations were repeated 3–4 times on different days from 0600–1800 hr in order to provide an accurate anthesis schedule. Similarly, the mature buds were also observed for recording the time of anther dehiscence. The presentation pattern of pollen was also investigated by recording how anthers dehisced and confirmed by observing the anthers under a 10x hand lens. Twenty-five mature but undehisced anthers were collected from different plants and placed in petri dishes. Later, every time, a single anther was taken out and placed on a clean microscope slide (75x25 mm) and dabbed with a needle in a drop of lactophenol-aniline-blue. The anther tissue was then observed under the microscope for pollen. The pollen mass was drawn into a band, and the total number of pollen grains was counted under a compound microscope (40x objective, 10x eye piece). The mean pollen output per anther was multiplied by the number of anthers in the flower for obtaining the mean number of pollen grains per flower. The characteristics of pollen grains were also noted down. The pollen-ovule ratio was determined by dividing the average number of pollen grains per flower by the number of ovules per flower (Cruden 1977). The presence of nectar was determined by observing the mature buds and open flowers. The volume of nectar from 20 flowers collected at random from five trees was measured. Then, the average volume of nectar per flower was determined and expressed in µl. The flowers used for this purpose were tied with bag at mature bud stage, opened after anthesis and the nectar squeezed into a micropipette for measuring the volume of the nectar. Nectar sugar concentration was determined using a Hand Sugar Refractometer (Erma, Japan). For the analysis of sugar types, the paper chromatography method described by Harborne (1973) was followed. The nectar was placed on Whatman No. 1 filter paper along with standard samples of glucose, fructose and sucrose. The paper was run ascendingly for 24 hours with a solvent system of n-butanol-acetone-water (4:5:1), sprayed with aniline oxalate spray reagent and dried at 1200C in an electric oven for 20 minutes for the development of spots from the nectar and the standard sugars. Then, the sugar types as well as the most dominant sugar type were documented based on the area and colour intensity of the spot. Nectar amino acid types were also noted down as per the paper chromatography method of Baker & Baker (1973). Nectar was spotted on Whatman No. 1 filter paper along with the standard samples of 19 amino acids, namely, alanine, arginine, aspartic acid, cysteine, cystine, glutamic acid, glycine, histidine, isolecuine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine and valine. The paper was run ascendingly in a chromatography chamber for 24 hours with a solvent system of n-butanol-glacial acetic acid-water (4:1:5). The chromatogram was detected with 0.2% ninhydrin reagent and dried at 850C in an electric oven for 15 minutes for the development of spots from the nectar and the standard amino acids. The developed nectar spots were compared with the spots of the standard amino acids to identify the types of amino acids. The stigma receptivity was observed visually and by H2O2 test. In the visual method, the stigma physical state (wet or dry) was considered to record the duration of receptivity. H2O2 test as given in Dafni et al. (2005) was followed for observing stigma receptivity period.
Pollinators
The bee species visiting the flowers were observed visually and by using a pair of Olympus Binoculars (PX35 DPSR Model). Their foraging activity was confined to the daytime and they were observed on a number of occasions for their foraging behaviour such as mode of approach, landing, probing behaviour, the type of forage they collect, contact with essential organs that result in pollination and inter-plant foraging activity in terms of cross-pollination. The foraging bees were captured from 1000–1200 hrs and brought to the laboratory. For each bee species, 10 specimens were captured and each specimen was washed first in ethyl alcohol, the contents stained with aniline-blue on a glass slide and observed under a microscope to count the number of pollen grains. Pollen loads on their corbiculae were separated prior to washing them. From this, the average number of pollen grains carried by each bee species was calculated to know their pollen carryover efficiency.
Breeding system
Mature flower buds of some inflorescences on different individuals were tagged and enclosed in butter paper bags for breeding experiments. The number of flower buds used for each mode of pollination is given in Table 1. The stigmas of flowers were pollinated with the pollen of the same flower manually by using a brush; they were bagged and followed to observe fruit set in manipulated autogamy. The flowers were fine-mesh bagged without hand pollination to observe fruit set in spontaneous autogamy. The emasculated flowers were hand-pollinated with the pollen of a different flower on the same plant; they were bagged and followed for fruit set in geitonogamy. The emasculated flowers were pollinated with the pollen of a different individual plant; they were bagged and followed for fruit set in xenogamy. If fruit set occurred, the percentage of fruit set was calculated for each mode. The flowers/inflorescences were tagged on different plant species prior to anthesis and followed for fruit/seed set rate in open-pollinations.
Fruiting ecology
The fruit maturation period was recorded by making field trips to the study sites during the whole period of fruiting. Careful observations were also made for fruit dispersal in ocean currents and tides. Fruit and seed characters were recorded in detail based on 50 fruits collected from 10 trees distributed randomly in the study sites.
Photography
Plant, flower and fruit details together with insect foraging activity on flowers were photographed with a Nikon D40X Digital SLR (10.1 pixel) and TZ240 Stereo Zoom Microscope with SP-350 Olympus Digital Camera (8.1 pixel).
RESULTS
Ecology
Scyphiphora hydrophyllacea is an erect, evergreen much-branched small tree. It is restricted to only one site, Kothapalem Reserve Forest in Coringa Mangrove forest (Image 1a). It grows in association with non-viviparous tree species, Lumnitzera racemosa Willd. (Combretaceae) and at ground level with Suaeda maritima (L.) Dumort., S. monoica Forssk. ex. J.F. Gmel and S. nudiflora (Willd.) Moq. (Chenopodiaceae) along the banks of tidal waterways that end up with the landward side. The soil here is muddy and sandy with salinity levels characteristic of oligo- and meso-saline zones. The soil texture is clay-rich with a mixture of sand and silt: clay 52.32%, sand 36.68% and silt 11%. The pH recorded is 7.74, electrical conductivity 2.98ds/m, organic carbon 0.85%, nitrogen 135kg/ha, phosphorous 13.49kg/ha and potassium 236kg/ha. In Scyphiphora hydrophyllacea, the leaf shedding is continuous but it is very prominent during the summer season. The flowering occurs almost throughout the year at the population level with profuse flowering during June–August and sparse flowering during the remaining period. In the case of sparse flowering, a few inflorescences with asynchronous bud development extend flowering on certain trees. The flowers are borne in dense clusters (cymes) in leaf axils on ca 15mm long peduncle and each cluster has an average of 23 flowers over a period of 5 days (Image 1b–e).

Flower morphology
The flowers are sessile, 14mm long, 6mm wide, white, odourless, and bisexual. The calyx is 5mm long, cup-shaped and crowned by four minute denticles. The corolla is tubular, white tinged somewhat with pink, 4mm long, with a rough-hairy mouth and terminated with four broadly elliptic lobes, and each lobe is 2mm long. Stamens are four, inserted in corolla just below the throat, partially to fully exserted, filaments short, anthers linear-sagittate (Image 2e), dorsifixed, and bifid at base. Ovary is 2-celled each with two ovules on axile placentation and attached in the middle of the septum with one erect and one pendulous ovule (Image 2j,k). The style is filiform terminated with wet bifid papillate stigma.

Floral biology
The buds develop and mature slowly (Image 1c-e). The mature buds open from 0600–1100 hr with a peak from 0900–1100 hr with the petals gradually exposing the still closed club-shaped stigma (Image 2a–c). The anthers dehisce introrsely during mature bud stage by longitudinal slits (Image 2d). The petals expand and reflex during which the style elongates, during which stage, the flower exposes the pollen distinctly. The pollen output per anther is 3440.8±236.03 and per flower is 13,760. Pollen grains are round in shape with three broad and long colpi, each domed with circular operculum, exine membrane with granular ornamentation, 24.9µm, creamy white, sticky initially and powdery later (Image 2f,g). The pollen-ovule ratio is 3440:1. The closed club-shaped stigma opens its lobes and reflex back on the 2nd day; it is then receptive and remains so until the evening of the 3rd day (Image 2h,i). During anthesis, pollen deposition occurs on the style and closed stigmatic lobes; pubescent hair situated at the corolla mouth facilitates this pollen deposition with certainty. In case of stigma, only the inner surface of lobes is receptive and there is a linear opening through the lobes. Then, pollen deposition occurs along the margins of stigmatic lobes and autogamy occurs on the 2nd day when the stigma is receptive. Nectar is secreted by the glandular disc situated at the base of the corolla tube. A flower produces 1.37±0.4 (Range 1.2–1.7) µl of nectar and the nectar sugar concentration is 26.5±1.4% (Range 21.3–29.6 %). The common sugars include sucrose, glucose and fructose with sucrose being dominant. The nectar contains four essential amino acids, arginine, histidine, threonine and tryptophan, and five non-essential amino acids, tyrosine, cystine, cysteine, alanine and proline. The anthers shrivel by noon of the day of anthesis and fall back along with the petals clearing the way for the forager. The corolla together with stamens and stigma wilts and remains in place until fruit drop whereas the calyx gradually bulges and encloses the gradually increasing ovary.
Breeding systems
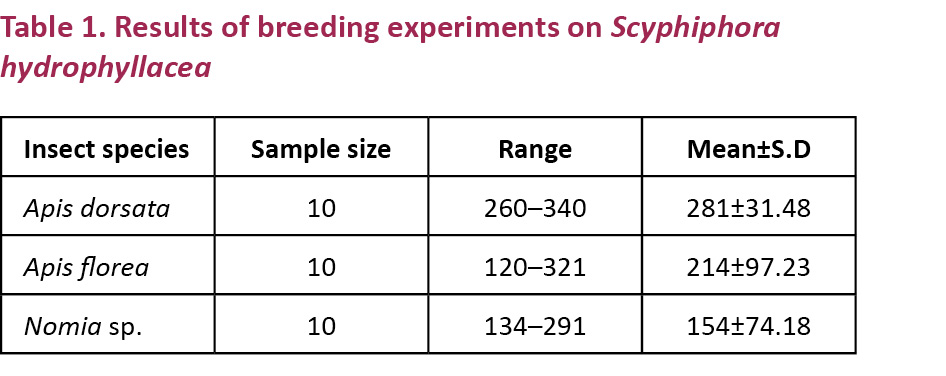
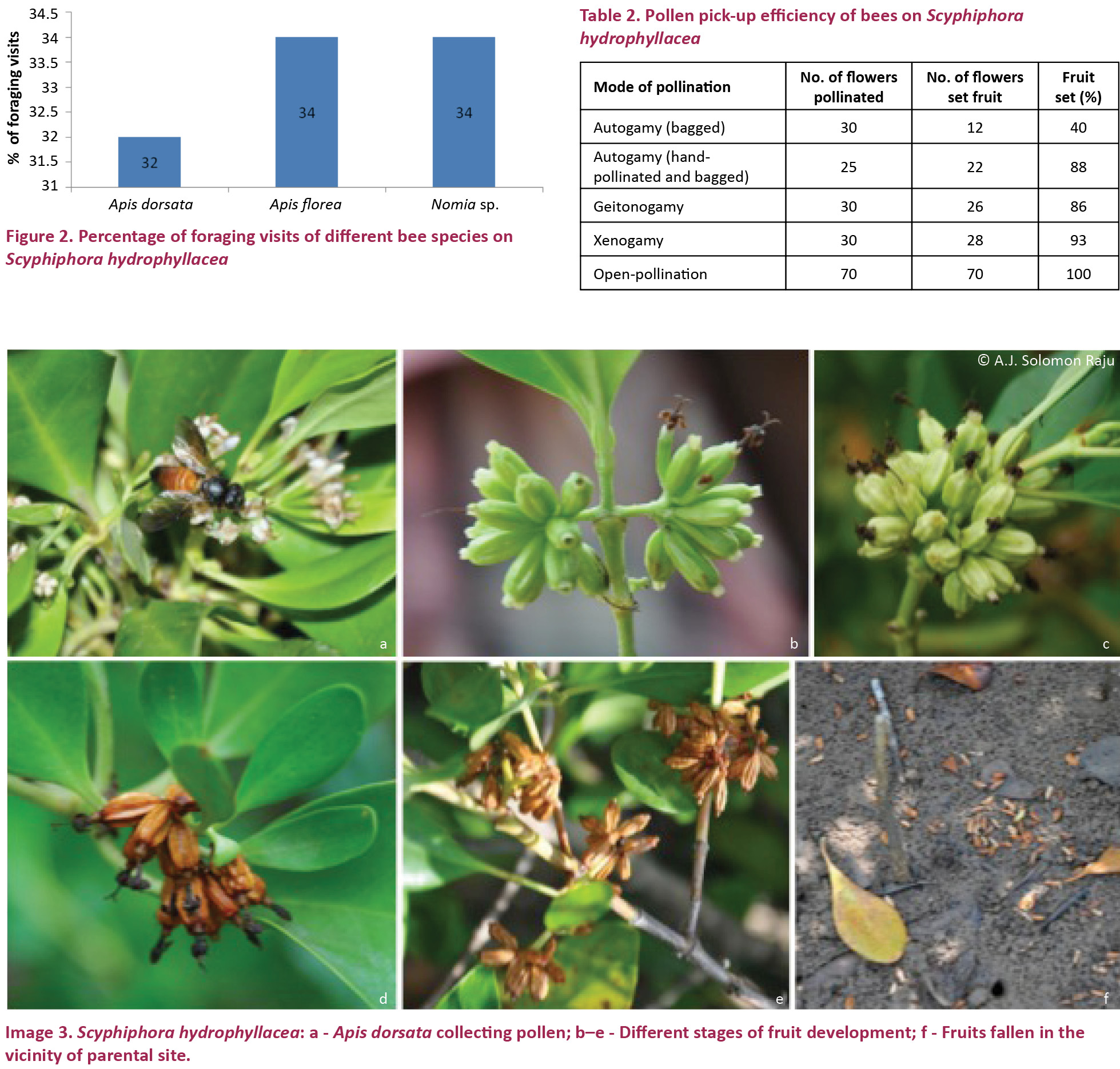
The results of breeding systems indicate that the flowers are self-compatible and self-pollinating. The fruit set rate varied with each mode of pollination. It is 40% in spontaneous autogamy, 88% in hand-pollinated autogamy, 86% in geitonogamy, 93% in xenogamy and 100% in open pollination (Table 1).
Pollinators
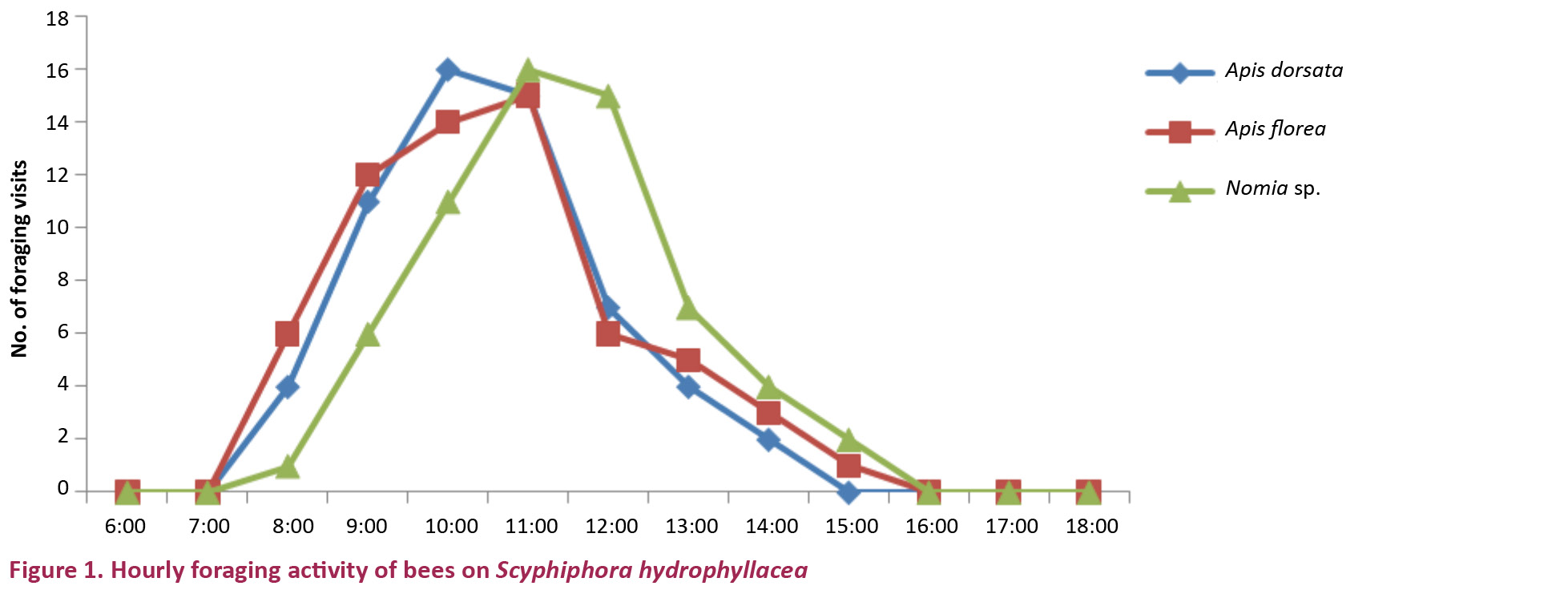
The flowers are unspecialized and the stamens and stigma are exposed when the petals reflex. They were foraged exclusively by bees during the day time consistently from 0800–1500 hr with more activity from 0900–1200 hr (Fig. 1). The bees were Apis dorsata (Image 3a), A. florea and Nomia sp. They have collected pollen and nectar in the same or in a different foraging visit simultaneously on the same or different flowering inflorescences. All the three bee species approached the flowers in an upright position, land on the reflexed petals and probe for nectar which is easily accessible due to the broad and spacious corolla tube. While doing so, A. dorsata invariably contacts both the stamens and stigma with its ventral side due to its larger body size. During pollen collection, its ventral side contacts the style and stigma. In both modes of forage collection, pollination is effected. A. florea and Nomia sp. did not contact the style and stigma while collecting the nectar but they made contact while collecting pollen indicating that only pollen collection activity of these result in pollination. Of the total foraging visits made by these bees, A. dorsata made 32%, A. florea and Nomia sp. each made 34% of visits (Fig. 2). Their body washings revealed the presence of pollen grains; the mean number varied from 154 to 281. A. dorsata carried 281±31.48, A. florea 214±97.23 and Nomia sp. 154±74.18 (Table 2). The results indicated that each insect species is a pollen carrier and the pollen carry-over capacity is related to the body size of the bee species. Although these bee species were the regular foragers during the profuse flowering period, they were not very consistent with numbers and visits. Lumnitzera racemosa as an associate plant, flowers profusely during the peak flowering period of S. hydrophyllacea and competes with the latter by attracting a variety of insects including bees, wasps and butterflies; of bees, all the three bee species recorded on S. hydrophyllacea also visit L. racemosa flowers alternately.
In S. hydrophyllacea, the flowers present sticky pollen initially during anthesis. At this time, the pollen sticks to the style and outer surface of the closed stigma. Gradually the pollen turns powdery and is driven by wind. As the plant’s occurrence is along the banks of tidal creeks and landward margins, as soon as the pollen becomes powdery, it is driven by the wind and in effect it settles on the closed un-receptive stigmas or open receptive stigmas of erect flowers. As a consequence, both self- and cross-pollination prevails in S. hydrophyllacea.
Fruiting behavior
Pollinated and fertilized flowers initiate fruit development immediately and takes 35–40 days to produce mature fruits in all inflorescences. Fruits are initially green, turn yellow as they ripen and finally shiny brown when fully mature (Image 3b–e). Fruit dispersal occurs as and when they mature but the main dispersal period is from late-July to mid-September which is related to the period of profuse flowering. The fruit is cylindrical, 8mm long, 6–8 ribbed along its length, corky inside and tipped with the remnants of the calyx. It is indehiscent and contains four cylindrical 1mm long sub-cylindrical seeds. Seeds remain within the fruit and are exposed after the decay of the fruit wall. But, seeds in all fruits are ill-developed and non-viable. Fruits are water-buoyant because of the corky and buoyant fruit wall and dispersed by tidal currents. The fruits become fibrous after floating and finally settle on the sticky silty mud within the parental site during low tide period (Image 3f). Seeds do not germinate and hence regeneration is totally absent.
DISCUSSION
Scyphiphora hydrophyllacea is a non-viviparous evergreen tree species. Different authors reported that it occurs in muddy, sandy and rocky substrates on the landward margin of mangroves or on the banks of tidal waterways. The sites where it grows are unsuitable for colonization by other mangrove species. It is not tolerant of lengthy periods of freshwater inundation and hence occupies sites that are frequently inundated by the tides (Heyne 1950; Tomlinson 1986; Wim et al. 2006; Tao & Charlotte 2011). This species occurs as one large population along the banks of tidal waterways that end up on the landward side of the Kothapalem site in Coringa Mangrove forest. In fact, this area is subjected to short periods of freshwater inundation. Wim et al. (2006) reported that S. hydrophyllacea flowers throughout the year. Almazol & Cervancia (2013) noted that in the Philippines, it flowers from February to June with the peak flowering period from March to May, and a few inflorescences in certain trees bloom after this regular flowering season. Fruiting occurs from April to October. Ramasubramanian et al. (2003) noted that both flowering and fruiting occurs in this species from May to August. The present study indicates that it flowers almost throughout the year at the population level with a concentrated flowering from June to August. The flowering outside this period is very sparse and occurs only in certain trees. Fruiting also occurs throughout the year depending on the production of flowers on the plant but peak fruiting occurs from July to September.
At the study site, profuse flowering occurs simultaneoulsy in both S. hydrophyllacea and Lumnitzera racemosa. This massive flowering pattern usually benefits individual species but S. hydrophyllacea is unable to attract a variety of insect species in the presence of L. racemosa and is in a disadvantageous position. In both S. hydrophyllacea and L. racemosa, the flowers are markedly protandrous and open at the same time during the forenoon period; the flowers are white, but in the case of S. hydrophyllacea, they are additionally tinged with pink, nectariferous, odourless. The stigma receptivity occurs on the 2nd and 3rd day in both the species. With identical structural and functional characteristics of flowers, S. hydrophyllacea is quite unsuccessful in competing with L. racemosa since the latter is regularly visited by different bees, wasps and butterflies. S. hydrophyllacea is pollinated by only three species of bees. These bees also visit L. racemosa alternately and it is certain that the stigmas of S. hydrophyllacea receive the pollen of the other species and hence such inter-specific foraging activity could affect the occurrence of compatible pollination, fertilization and subsequent formation of fruits with viable seeds in this species. The flowers of S. hydrophyllacea are 4-ovuled and all fruits produce defective or non-viable seeds due to which regeneration is totally absent.
Puff et al. (1996) reported that Secondary Pollen Presentation (SPP) occurs widely in all the sub-families of Rubiaceae. They recognized four types of SPP based on the pollen presenting area and receptive surface of style and stigma. In the first type, pollen deposition occurs on the style only and its deposition is strictly on non-receptive surfaces. In the second type, pollen deposition occurs on the style and outside of the stigma lobes; pollen is solely deposited on non-receptive surfaces. In the third type, pollen deposition occurs on the outer side of the stigma while in the fourth type, it occurs exclusively, largely or partly on the receptive surface of the stigma. Almazol & Cervancia (2013) mentioned that in S. hydrophyllacea, selfing may be promoted by the adherence of pollen on the outside of the style. Puff & Rohrhofer (1993) reported that the flowers of S. hydrophyllacea possess an “ixoroid” pollination mechanism representing the second type of SPP in which the flowers are protandrous and deposit the pollen on the outside of the stigmas and style for dispersal. The present study confirms the same with a deviation that during anthesis, pubescent hair situated at the corolla mouth facilitates brushing of style and stigma against the dehisced anthers with certainty. In the stigma, the outer surface is non-receptive while its inner surface is receptive on the 2nd and 3rd day of flowering. Self-pollen deposition occurs along the margins of stigmatic lobes and part of it enters through a linear opening between them facilitating autogamy when the stigma is receptive. Pollen is viable on the 2nd and even on 3rd day of the flower’s life and it is confirmed by the occurrence of fruit set in bagged flowers. But, fruit set in this mode is not one hundred percent. But, Almazol & Cervanicia (2013) reported that fruit set is 100% in bagged and un-bagged treatments of S. hydrophyllacea; all fruits in un-bagged flowers mature while fruits from bagged flowers are an indication of self-fertility but they display high abortion rate indicating some inbreeding depression or poor nutrition. Although these studies differ in the percentage of fruit set, they commonly indicate that fruit set is not essentially vector-dependent. In addition to SPP, wind also transfers pollen and effects self- and cross-pollination since the habitat of the species is windy most of the time, day and night.
S. hydrophyllacea exhibits mixed mating system because of the occurrence of fruit set through all modes of pollination. The pollen-ovule ratio facilitates the function of this mating system (Cruden 1977). The flower sexual system is indicative of temporal dioecy since the flowers are staminate on the day of anthesis by marked protandry and pistillate on 2nd and 3rd day. Self-pollination within the flower is not vector-dependent while self-pollination between flowers on the same or different individuals of this species requires external agents. At this juncture, different authors stated that it is entomophilous or insect- or bee-pollinated (Tomlinson 1986; Wheeler et al. 1992; Selvam and Karunagaran 2004; Wim et al. 2006; Almazol & Cervancia 2013). The study by Almazol & Cervancia (2013) indicated that the plant is pollinated by a total of 15 insect species out of which only three were bee species, namely, Xylocopa sp., Apis dorsata and Tetragonula biroi. In this study, S. hydrophyllacea is exclusively pollinated by three bee species only viz, Apis dorsata, A. florea and Nomia sp.; they use the flowers as a pollen and nectar source; the latter is an important source of sugars and certain essential as well as non-essential amino acids (DeGroot 1953). The peduncle of the inflorescence keeps the flowers in an almost erect position and supports the flowers to hold the larger foragers such as Apis dorsata. The reflexed petals serve as a landing platform for the large insects (Almazol & Cervancia 2013). The floral traits of S. hydrophyllacea mentioned earlier are suggestive of entomophily but in this case, melittophily. These bees with their intra-specific foraging activity at individual and population level bring about both self- and cross-pollination.
In S. hydrophyllacea, self-pollinating ability without vectors is important to colonize an area and establish population in isolated localities. The vector-mediated pollination facilitates the occurrence of genetic variation that is essentially required for adaptation to changing edaphic and physical environment. The mixed mating system is advantageous for the plant to adapt itself to the characteristic harsh environments of mangroves. Despite the ability to set fruit to one hundred percent through self- and cross-pollination, the plant is unable to regenerate itself in the study site due to a total absence of seed germination. Fruits are indehiscent and float as such and are dispersed by the tidal current. But, most of the fruits finally settle on sticky silty mud within the parental site. The chemical characters of the soil indicate that pH is slightly alkaline, electrical conductivity high, organic carbon optimal, nitrogen extremely low, phosphorous optimal and potassium high. The soil texture characteristically is clay with a mixture of sand and silt. These physico-chemical characters suggest that the soil is poor in nitrogen with high organic carbon and has an injurious level of electrical conductivity suggesting that it may have a significant impact on the growth rate, physiology and genetic material of the standing stock of the plant. The soil characters are not constant since they are regularly subjected to tidal flushes or inundation due to which in situ accumulation of soil is not possible. Almazol & Cervancia (2013) noted that seed germination occurs from the fruits of both bagged and un-bagged flowers of S. hydrophyllacea in the Philippines. They also noted that seed germination is significantly higher from the fruits of un-bagged flowers but overall percent of germination is below 20%. Hettiarachchi et al. (2002) reported that in Sri Lanka S. hydrophyllacea produces fruits but seedlings and young plants are absent. It produces very low percentage of seed bearing fruits and inability to produce healthy seedlings and hence is highly threatened throughout the world. This is attributed to genetic disorder in seed due to inbreeding depression in isolated small populations. Presence of self-sterility and the absence of pollinators might be some other reasons. The present study supports this hypothesis but suggests molecular studies for confirmation. Further, the existing population of S. hydrophyllacea at the study site may disappear soon if it is not protected from human activities due to the lack of regeneration. It is used locally as an important source of fuel wood especially during the summer season and land use changes are also currently taking place in the area. Previous reports and the present study indicate that it is an uncommon species and threatened due to its use by locals primarily as fuel wood and failure of seed regeneration in almost all areas of its distribution. Therefore, it is suggested that further studies should focus on how to restore and expand populations of this species in its natural areas throughout its distribution range.
REFERENCES
Almazol, A.E. & C.R. Cervancia (2013). Floral biology and pollination of three mangrove species (Aegiceras floridum Roem. & Schults., Scyphiphora hydrophyllacea Gaertn.f., and Xylocarpus granatum Koen.) in Pagbilao Mangrove forest, Quezon Province, Philippines. Journal of Nature Studies 12: 39–47.
Baker, H. G. & I. Baker (1973). Some anthecological aspects of evolution of nectar-producing flowers, particularly amino acid production in nectar, pp. 243–264. In: Heywood, V.H. (ed.). Taxonomy and Ecology. Academic Press, London.
Cruden, R.W. (1977). Pollen-ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46; http://dx.doi.org/10.2307/2407542
Dafni, A., P.G. Kevan & B.C. Husband (2005). Practical Pollination Biology. Enviroquest Ltd., Cambridge, Canada, 583pp.
DeGroot, A.P. (1953). Protein and amino acid requirements of the Honey Bee (Apis mellifera L. Physiologia Comparata et Oecologia 3: 197–285.
Ellison, J., N.E. Koedam, Y. Wang, J. Primavera, O. Jin Eong, J.W.H. Yong & V.N. Nam (2010). Scyphiphora hydrophyllacea. In: IUCN 2013 IUCN Red List of Threatened Species. Version 2013.2. www.iucnredlist.org. 5th July 2014.
Harborne, J.B. (1973). Phytochemical Methods. Chapman and Hall, London, 288pp.
Heyne, K. (1950). De Nuttige planten van Indonesie (The useful plants of Indonesia). 3rd Edition, 2 Volumes. W. van Hoeve-Gravenhage, the Netherlands/Bandung, Indonesia, 1660pp.
Hettiarachchi, P. L., P.A.G.W. Premathilake & S. Hettiarachchi (2002). Vegetative propagation of Scyphiphora hydrophyllacea Gaertn F. for conservation. Forestry and Environment Symposium 2002 of the Department of Forestry and Environmental Science, University of Sri Jayewardenepura, Sri Lanka.
Puff, C. & U. Rohrhofer (1993). The character states and taxonomic position of the monotypic mangrove genus Scyphiphora (Rubiaceae). Opera Botanica Belgica 6: 43–172.
Puff, C., E. Robbrecht., R. Buchner & P. De Block (1996). A survey of secondary pollen presentation in the Rubiaceae. Opera Botanica Belgica 7: 369–402.
Ramasubramanian, R., T. Ravishankar & D. Sridhar (2003). Mangroves of Andhra Pradesh. Identification and Conservation Manual. M.S. Swaminathan Research Foundation, 67pp.
Rao, T.A., S. Molur & S. Walker (eds.)(1998). Report of the workshop “Conservation Assessment and Management Plan for Mangroves of India” (BCPP Endangered Species Project). Zoo Outreach Organization, Conservation Breeding Specialist Group India, Coimbatore, 106pp.
Selvam, V. & V.M. Karunagaran (2004). Ecology and Biology of Mangroves. M.S. Swaminathan Research Foundation, Chennai, 61pp.
Tao, C. & M.T. Charlotte (2011). Scyphiphora, S. 323 - Gattung und art - textgleich online wie gedrucktes Werk. In: Zheng-yi, W., P.H. Raven & H. Deyuan (eds.). Flora of China, Volume - Cucurbitaceae through Valerianaceae, with Annonaceae and Berberidaceae. Science Press und Missouri Botanical Garden Press, Beijing und St. Louis.
Tomlinson, P.B. (1986). The Botany of Mangroves. Cambridge University Press, 419pp.
Wheeler, J.R., B.L. Rye., B.L. Koch & A.J.G. Wilson (1992). Western Australian Herbarium. Flora of the Kimberley Region. Western Australian Herbarium, Como, W.A, 1392pp.
Wim, G., W. Stepha., Z. Max & S. Liesbeth (2006). Mangrove Guidebook for Southeast Asia. FAO Regional Office, Bangkok, 186pp.